Global Pharmacovigilance and Drug Safety Software Market Is Expected To Reach Around USD 223 Million By 2025
14-Mar-2019 | Zion Market Research
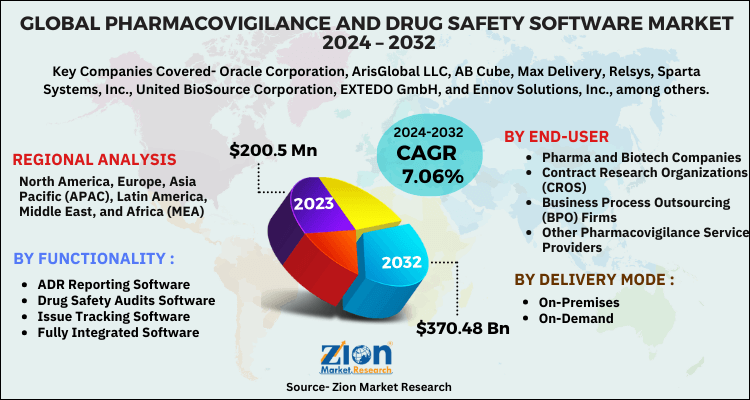
Zion Market Research has published a new report titled “Pharmacovigilance and Drug Safety Software Market by Functionality (ADR Reporting Software, Drug Safety Audits Software, Issue Tracking Software, and Fully Integrated Software), by Delivery Mode (On-Premises and On-Demand), and by End-User (Pharma & Biotech Companies, Contract Research Organizations (CROS), Business Process Outsourcing (BPO) Firms, and Other Pharmacovigilance Service Providers): Global Industry Perspective, Comprehensive Analysis, and Forecast, 2018–2025”. According to the report, the global pharmacovigilance and drug safety software market was valued at approximately at USD 141 million in 2018 and is expected to generate around USD 223 million by 2025, at a CAGR of around 6.7% between 2019 and 2025.
According to the World Health Organization (WHO), pharmacovigilance is the science and activities related to the understanding, evaluation, detection, and prevention of adversarial reactions to medications or problems related to medicine. The scope and definition of pharmacovigilance have evolved to identify the significance of a systems approach for improving and monitoring the harmless and safe use of medicines.
Browse the full “Pharmacovigilance and Drug Safety Software Market by Functionality (ADR Reporting Software, Drug Safety Audits Software, Issue Tracking Software, and Fully Integrated Software), by Delivery Mode (On-Premises and On-Demand), and by End-User (Pharma & Biotech Companies, Contract Research Organizations (CROS), Business Process Outsourcing (BPO) Firms, and Other Pharmacovigilance Service Providers): Global Industry Perspective, Comprehensive Analysis, and Forecast, 2018–2025” Report at https://www.zionmarketresearch.com/report/pharmacovigilance-drug-safety-software-market
Pharmacovigilance and drug safety software or tools aid in classifying, reviewing, and forming pharmacovigilance data. This software also helps in the formation of repots related to adverse medical events. Additionally, the pharmacovigilance software is majorly used by biotech and pharmaceutical organizations, business process outsourcing firms (BPOs), contract research organizations (CROs), and many other pharmacovigilance service providers. Pharmacovigilance globalization with extensive availability and access of the internet is likely to fuel the global pharmacovigilance and drug safety software market in the future. Moreover, the augmenting adoption of pharmacovigilance software by numerous outsourcing companies will also serve as a huge growth opportunity for the global pharmacovigilance and drug safety software market over the forecast timespan.
The global pharmacovigilance and drug safety software market is segmented on the basis of functionality, delivery mode, and end-user. Based on functionality, the market is segmented into drug safety audits software, ADR reporting software, fully integrated software, and issue tracking software. The fully integrated software segment is expected to register the highest growth over the forecast time period, owing to the increasing need to prevent errors in database management regarding medicines.
By delivery mode, the market is segmented into on-premises and on-demand. The on-premises segment is anticipated to hold the largest market share, owing to its rising adoption by large pharmaceutical companies as it encompasses the installation of solutions and services on desktops within the organizational premises. Alternatively, the on-demand segment is anticipated to register the highest CAGR globally.
By end-user, the market is segmented into contract research organizations (CROS), business process outsourcing (BPO) firms, pharma and biotech companies, and other pharmacovigilance service providers. The pharma and biotech companies are estimated to dominate the global pharmacovigilance and drug safety software market over the forecast timeline, owing to the high software adoption of to aid clinical trial activities and decrease medical expenditures. The contract research organizations (CRO’S) are anticipated to show the highest CAGR in the global pharmacovigilance and drug safety software market, owing to increasing outsourcing activities in pharmaceutical and biotech companies.
By region, North America is anticipated to lead the global pharmacovigilance and drug safety software market over the forecast period, owing to the high adoption of pharmacovigilance and drug safety software and favorable government regulations. Europe was the second largest market in 2018, followed by the Asia Pacific. The Asia Pacific pharmacovigilance and drug safety software market is anticipated to record lucrative growth over the projected time period, subject to an increasing number of clinical trials being conducted in the region and the considerable cost-saving benefits.
Some key players of the global pharmacovigilance and drug safety software market include Oracle Corporation, ArisGlobal LLC, AB Cube, Max Delivery, Relsys, Sparta Systems, Inc., United BioSource Corporation, EXTEDO GmbH, and Ennov Solutions, Inc., among others.
This report segments the global pharmacovigilance and drug safety software market into:
Global Pharmacovigilance and Drug Safety Software Market: Functionality Analysis
- ADR Reporting Software
- Drug Safety Audits Software
- Issue Tracking Software
- Fully Integrated Software
Global Pharmacovigilance and Drug Safety Software Market: Delivery Mode Analysis
- On-Premises
- On-Demand
Global Pharmacovigilance and Drug Safety Software Market: End-User Analysis
- Pharma and Biotech Companies
- Contract Research Organizations (CROS)
- Business Process Outsourcing (BPO) Firms
- Other Pharmacovigilance Service Providers
Global Pharmacovigilance and Drug Safety Software Market: Regional Analysis
- North America
- The U.S.
- Europe
- UK
- France
- Germany
- Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- The Middle East and Africa
About Us:
Zion Market Research is an obligated company. We create futuristic, cutting-edge, informative reports ranging from industry reports, the company reports to country reports. We provide our clients not only with market statistics unveiled by avowed private publishers and public organizations but also with vogue and newest industry reports along with pre-eminent and niche company profiles. Our database of market research reports comprises a wide variety of reports from cardinal industries. Our database is been updated constantly in order to fulfill our clients with prompt and direct online access to our database. Keeping in mind the client’s needs, we have included expert insights on global industries, products, and market trends in this database. Last but not the least, we make it our duty to ensure the success of clients connected to us—after all—if you do well, a little of the light shines on us.
Contact Us:
Zion Market Research
244 Fifth Avenue, Suite N202
New York, 10001, United States
Tel: +49-322 210 92714
USA/Canada Toll-Free No.1-855-465-4651
Email: sales@zionmarketresearch.com
Website: https://www.zionmarketresearch.com
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed


