Global Phenylketonuria Treatment Market Set for Rapid Growth, to reach Value around USD 925.0 Million by 2025
25-Oct-2019 | Zion Market Research
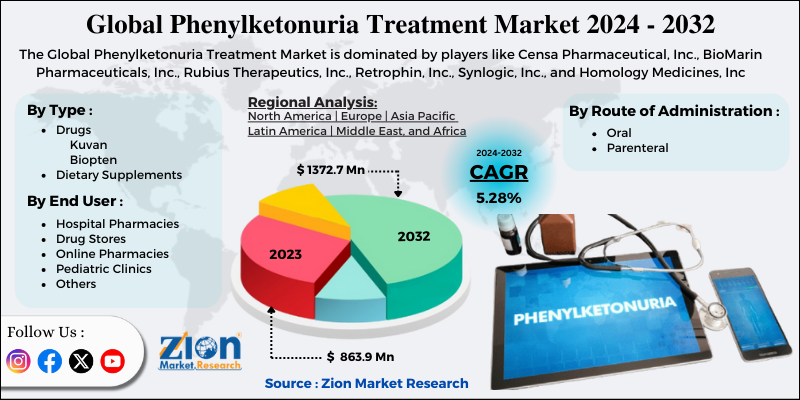
Zion Market Research has published a new report titled “Phenylketonuria Treatment Market by Type (Drugs [Kuvan, and Biopten], and Dietary Supplements), by Route of Administration (Oral and Parenteral), and by End User (Hospital Pharmacies, Drug Stores, Online Pharmacies, Pediatric Clinics, and Others): Global Industry Perspective, Comprehensive Analysis and Forecast, 2019 – 2025”. According to the report, global demand for phenylketonuria treatment market was valued at approximately USD 434.5 million in 2018, and is expected to generate revenue of around USD 925.0 million by end of 2025, growing at a CAGR of around 11.4% between 2019 and 2025.
Browse the full “Phenylketonuria Treatment Market: by Type (Drugs [Kuvan, and Biopten], and Dietary Supplements), by Route of Administration (Oral and Parenteral), and by End User (Hospital Pharmacies, Drug Stores, Online Pharmacies, Pediatric Clinics, and Others): Global Industry Perspective, Comprehensive Analysis and Forecast, 2019 – 2025” Report at https://www.zionmarketresearch.com/report/phenylketonuria-treatment-market
A genetic disorder is in which levels of phenylalanine increase in the blood is known as Phenylketonuria (PKU). PKU can cause seizures, delayed development, behavioral problems, and psychiatric disorders if it is not treated on time. Kids suffering from classic phenylketonuria are at high risk of brain damage and require treatment. Phenylketonuria varies from mild to severe forms, but variant PKU is a lesser severe disease form whereas, classic PKU is considered among severe. Growing prevalence of PKU globally is probable to accelerate the market growth for phenylketonuria treatment. According to stats by Genetic Home Reference, in the U.S. 1 in 10,000 cases of PKU occur in newborns.
Presence of pipeline drugs, like SYNB1618, CNSA-001, and RTX-134 are likely to fuel the phenylketonuria treatment market. CNSA-001 has accomplished its Phase I study successfully and has also initiated Phase II clinical trial likewise; SYNB1618 is under Phase 2a clinical trial currently and has also received FDA’s fast-track designation. Moreover, growing development of new drugs and commercialization is expected to boost the market growth for PKU globally.
The market for phenylketonuria treatment is segmented based on route of administration, type, end user, and region. Based on route of administration the market is divided into parenteral and oral wherein the segment for oral route of administration held major share in 2018. This route of administration is preferred by patients due to its convenience and ease of administration since parenteral infusions are difficult to administer. End user market is segmented into pediatric clinics, drug stores, hospital pharmacies, online pharmacies, and others. The segment for hospital pharmacies held major share of the market in 2018.
Based on type the market is segmented into dietary supplements, and drugs. The market for drugs segment in PKU is further segmented into biopten, and kuvan. The segment for Kuvan held major share in 2018. Kuvan oral drug is manufactured by BioMarin Pharmaceutical, Inc. The drug got approval in the U.S. in 2007 and in 2008 in Europe. The patent of this drug was expired in the year 2015 in the U.S. and in Europe is about to expire in the year 2020. Nevertheless, Par Pharmaceuticals and BioMarin have entered into a settlement agreement to launch generic version of Kuvan powder and tablets form.
Regional segmentation includes the current and forecast demand for Asia Pacific, North America, Latin America, Europe, and Middle East & Africa with its further bifurcation into major countries. North America accounted major share of the market in 2018, due to favorable regulations to treat PKU and growing government initiatives in this region. Moreover, continuous commercialization and research & development of novel drugs is also likely to propel the market for PKU treatment in this region.
Some of the players included in PKU treatment market are BioMarin Pharmaceuticals, Inc., Rubius Therapeutics, Inc., Synlogic, Inc., Censa Pharmaceutical, Inc., Retrophin, Inc., and Homology Medicines, Inc.
The report segments the phenylketonuria treatment market as follows:
Global Phenylketonuria Treatment Market: Type Segment Analysis
- Drugs
- Kuvan
- Biopten
- Dietary Supplements
Global Phenylketonuria Treatment Market: Route of Administration Segment Analysis
- Oral
- Parenteral
Global Phenylketonuria Treatment Market: End User Segment Analysis
- Hospital Pharmacies
- Drug Stores
- Online Pharmacies
- Pediatric Clinics
- Others
Global Phenylketonuria Treatment Market: Regional Segment Analysis
- North America
- U.S.
- Europe
- UK
- France
- Germany
- Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- Middle East and Africa
About Us:
Zion Market Research is an obligated company. We create futuristic, cutting-edge, informative reports ranging from industry reports, the company reports to country reports. We provide our clients not only with market statistics unveiled by avowed private publishers and public organizations but also with vogue and newest industry reports along with pre-eminent and niche company profiles. Our database of market research reports comprises a wide variety of reports from cardinal industries. Our database is been updated constantly in order to fulfill our clients with prompt and direct online access to our database. Keeping in mind the client’s needs, we have included expert insights on global industries, products, and market trends in this database. Last but not the least, we make it our duty to ensure the success of clients connected to us—after all—if you do well, a little of the light shines on us.
Contact Us:
Zion Market Research
244 Fifth Avenue, Suite N202
New York, 10001, United States
Tel: +49-322 210 92714
USA/Canada Toll-Free No.1-855-465-4651
Email: sales@zionmarketresearch.com
Website: https://www.zionmarketresearch.com
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed


