Global Regulatory Affairs Outsourcing Market Set for Rapid Growth, to Reach around USD 21.21 Billion By 2032
17-Jun-2024 | Zion Market Research
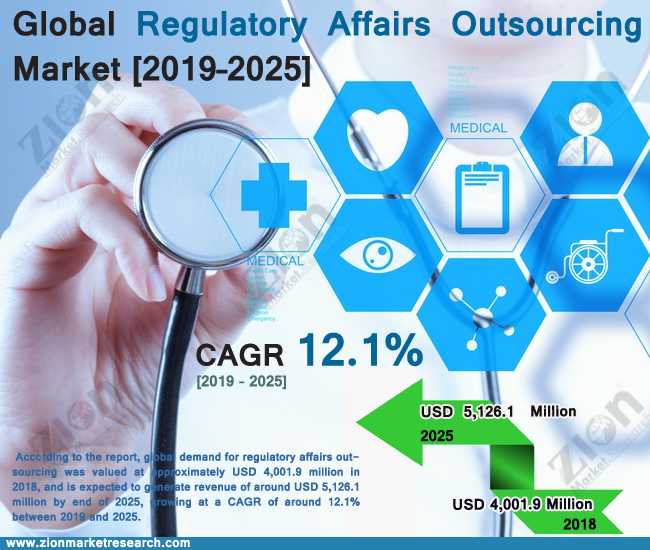
Zion Market Research has published a new report titled “Regulatory Affairs Outsourcing Market By Services (Regulatory Consulting, Legal Representation, Regulatory Writing & Publishing, Product Registration & Clinical Trial Application, and Other Regulatory Services), and By Region: Global Industry Perspective, Comprehensive Analysis and Forecast, 2024 - 2032” According to the report, the global regulatory affairs outsourcing market size was valued at approximately USD 10.18 billion in 2023 and is predicted to reach around USD 21.21 billion by the end of 2032, expanding at a CAGR of around 8.5% from 2024 to 2032..
Browse the full “Regulatory Affairs Outsourcing Market By Services (Regulatory Consulting, Legal Representation, Regulatory Writing & Publishing, Product Registration & Clinical Trial Application, and Other Regulatory Services), and By Region: Global Industry Perspective, Comprehensive Analysis and Forecast, 2024 - 2032” Report at https://www.zionmarketresearch.com/report/regulatory-affairs-outsourcing-industry
Outsourcing regulatory affairs to contract research organizations exercises provides reduced regulatory submission time, cost efficiency, and quality control in the manufacturing process. These components are expected to drive the growth over the time frame of the conjecture. Large organizations tackle capacity issues and absence of knowledge in their inner departments by outsourcing to smaller mid-sized enterprises. It also wipes out the remaining additional burden and combines administrative offices to avoid duplication of job. Regulatory affairs include exercises such as approvals drug shipment for clinical trials, drug master documents, labeling, and technical writing, reporting of severe adverse events, chemistry assessment, maintenance of Investigational New Drug (IND), eCTD conversion, query and controls management (CMC).
Increased R&D operations especially in the life science sector are expected to drive development by decreasing the general approval process by decreasing delays in legislative filings and improving Return on Investment (ROI) and cost effectiveness. In addition, favorable legislative mandates are expected during the forecast period to drive adoption. In August 2018, for example, the U.S. ICH E17 guidance on multi-regional clinical trials has been released by the Food and Drug Administration (FDA). It is anticipated that this initiative will introduce a single protocol to worldwide clinical trials, presented by various officials in distinct areas. Outsourcing of regulatory affairs has experienced few significant occurrences in the sector. In August 2018, for instance, U.K. based Syneos Health acquired Kinapse, Pharmacovigilance consulting and post-market regulatory services provider, was purchased. The firm seeks to double its consulting footprint in Europe and related business services with this purchase.
On the basis of service the market for regulatory affairs outsourcing is segmented into publishing & regulatory writing, legal representation, clinical trial application and product registration, regulatory consulting, and other regulatory services. Regulatory writing and publication retained biggest share of the market in 2023 and is likely to lead continue to lead. However, legal representation over the forecast period is expected to grow at high CAGR. Increasing demand for legal representatives in developed regions like North America and Europe are gaining traction for market permission ideas and businesses that want to set up a base in other nations concerned.
Europe, North America, Latin America, Asia Pacific, and the Middle East and Africa are the major regional segments of the global regulatory affairs outsourcing market. North America is anticipated to contribute significantly to worldwide market development. Global pharmaceutical and life science businesses, solid regulators like FDA, and talent pool accessibility will lead to overall development. In the coming years, Asia Pacific is expected to be the fastest growing region.
Some of the players included in the regulatory affairs outsourcing market are ICON Plc, Accell Clinical Research, Pharmaceutical Product Development (PPD) LLC, PARAXEL International Corporation, Covance, Inc., Genpact Ltd., Charles River Laboratories International, Inc., Medpace, Inc., Wuxi AppTec, and Criterium, Inc.
This report segments the global regulatory affairs outsourcing market as follows:
Global Regulatory Affairs Outsourcing Market: Services segment Analysis
- Regulatory Consulting
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Application
- Other Regulatory Services
Global Regulatory Affairs Outsourcing Market: Regional Segment Analysis
- North America
- U.S.
- Europe
- UK
- France
- Germany
- Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- Middle East and Africa
About Us:
Zion Market Research is an obligated company. We create futuristic, cutting-edge, informative reports ranging from industry reports, the company reports to country reports. We provide our clients not only with market statistics unveiled by avowed private publishers and public organizations but also with vogue and newest industry reports along with pre-eminent and niche company profiles. Our database of market research reports comprises a wide variety of reports from cardinal industries. Our database is been updated constantly in order to fulfill our clients with prompt and direct online access to our database. Keeping in mind the client’s needs, we have included expert insights on global industries, products, and market trends in this database. Last but not the least, we make it our duty to ensure the success of clients connected to us—after all—if you do well, a little of the light shines on us.
Contact Us:
Zion Market Research
244 Fifth Avenue, Suite N202
New York, 10001, United States
Tel: +49-322 210 92714
USA/Canada Toll-Free No.1-855-465-4651
Email: sales@zionmarketresearch.com
Website: https://www.zionmarketresearch.com
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed


