Medical Device CRO Market Size, Share, Growth, Opportunities 2034

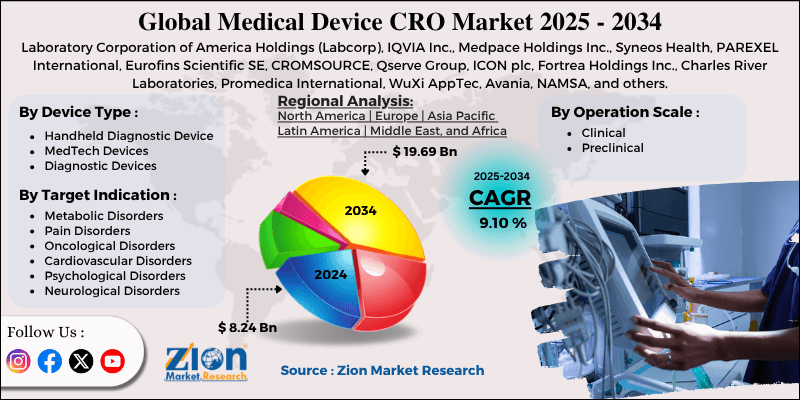
Medical Device CRO Market By Device Type (Handheld Diagnostic Device, MedTech Devices, Diagnostic Devices, and Others), By Operation Scale (Clinical and Preclinical), By Target Indication (Metabolic Disorders, Pain Disorders, Oncological Disorders, Cardiovascular Disorders, Psychological Disorders, Neurological Disorders, and Others), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 8.24 Billion | USD 19.69 Billion | 9.10% | 2024 |
Medical Device CRO Industry Prospective:
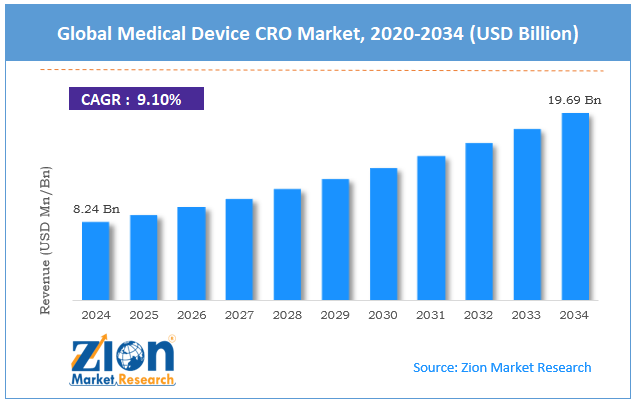
The global medical device CRO market size was worth around USD 8.24 billion in 2024 and is predicted to grow to around USD 19.69 billion by 2034, with a compound annual growth rate (CAGR) of roughly 9.10% between 2025 and 2034.
Medical Device CRO Market: Overview
Medical device contract research organizations (CRO) are strategic partners to medical device companies. These entities are responsible for assisting medical device developers in innovating and commercializing a novel product in the healthcare market. CROs deliver critical services in terms of conducting important clinical trials and also managing regulatory requirements. Furthermore, most CROs also deliver post-market analysis for the clients. Medical device CROs are experts in providing highly comprehensive services throughout product development. Medical device contract research organizations provide clinical trial design & management, regulatory assistance, and post-market data management & analysis.
The demand for medical device CRO is expected to grow at a steady pace during the projection period. The growing complexities in developing medical devices across the world are propelling demand for efficient CROs. On the other hand, the high cost associated with leveraging CRO services may impact overall revenue in the industry during the projection period.
Key Insights:
- As per the analysis shared by our research analyst, the global medical device CRO market is estimated to grow annually at a CAGR of around 9.10% over the forecast period (2025-2034)
- In terms of revenue, the global medical device CRO market size was valued at around USD 8.24 billion in 2024 and is projected to reach USD 19.69 billion by 2034.
- The medical device CRO market is projected to grow at a significant rate due to the changing global landscape concerning medical device regulations.
- Based on the device type, the medtech devices segment is growing at a high rate and will continue to dominate the global market as per industry projections.
- Based on the target indication, the oncological disorders segment is anticipated to command the largest market share.
- Based on region, North America is projected to dominate the global market during the forecast period.
Medical Device CRO Market: Growth Drivers
Changing global landscape concerning medical device regulations to propel market expansion
The global medical device CRO market is expected to be driven by the changing landscape across the globe concerning the regulatory framework for medical devices. In February 2025, Africa Centers for Disease Control and Prevention (Africa CDC) and the African Union Development Agency – New Partnership for Africa’s Development (AUDA-NEPAD) announced that they had signed a new Memorandum of Understanding (MoU) among Africa’s World Health Organization (WHO) Maturity Level 3 National Regulatory Authorities (NRAs). The MoU is a key milestone that aims to strengthen the region's regulatory systems by encouraging reliance and collaboration on regulatory decisions concerning medical products.
Similarly, other continents and regions are witnessing the evolution of regulatory guidelines covering several aspects of medical device production and final application. As international legal frameworks surrounding medical device production become more complex, CROs can prove beneficial to medical equipment makers. Contract research organizations have required expertise to ease the process for clients operating on a global scale.
Increasing demand for next-generation medical devices worldwide to promote market expansion
The current healthcare infrastructure is overburdened in the backdrop of an exponentially growing rate of patients. The global healthcare industry is facing an urgent demand to develop novel devices for improved diagnosis, treatment, prevention, monitoring, and post-operative care. The emergence of cutting-edge medical devices, such as wearables and implants, has further strengthened the urgency to develop more effective medical equipment. For instance, the current trend associated with research & development of multi-cancer early detection technologies may pave the way for increased revenue for leaders in the global medical device CRO market.
Medical Device CRO Market: Restraints
High expenses associated with CRO services impede market growth trends
The global medical device CRO industry is expected to be restricted due to the high expenses associated with these services. For instance, the average cost of a small-scale feasibility test conducted by a medical device CRO may range between USD 100,000 and USD 500,000. The cost may increase further depending on the scale of the services and expertise required. The high expenses can deter new players with limited budgets from taking advantage of the market's offerings.
Medical Device CRO Market: Opportunities
Increasing strategic partnerships between market players to generate growth opportunities
The global medical device CRO market is anticipated to gain growth opportunities due to the surge in strategic partnerships among stakeholders across the globe. For instance, in November 2025, Novotech, a leading full-service CRO, announced a novel partnership with Acrostar. The latter is a provider of Site Management Organization (SMO) services. The companies have signed an MoU with Kyungpook National University Hospital (KNUH). The partnership will encourage clinical trials across prestigious institutes in KNUH, including the main campus of the university and the Advanced Clinical Trials Center.
In September 2024, Eclever MedTech, a CRO specializing in medical devices & in vitro diagnostics, announced its selection by Unither Pharmaceuticals. Eclever will now lead the clinical studies on emerging medical devices by Unither. In October 2024, Namsa and Terumo entered a strategic outsourcing partnership. The former is a leading contract research organization while the latter develops medical devices. The collaboration aims to accelerate regulatory approvals and commercialization of products offered by Terumo.
Medical Device CRO Market: Challenges
Risk of loss of proprietary information and limited supply of skilled resources to challenge market expansion
The global medical device CRO industry is projected to be challenged by the risk of proprietary information loss. CRO companies deal with multiple clients across global regions and are at a higher risk of compromising the integrity of intellectual property of their clients. Moreover, limited access to skilled workforce in certain parts of the world may further affect market revenue.
Medical Device CRO Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Medical Device CRO Market |
| Market Size in 2024 | USD 8.24 Billion |
| Market Forecast in 2034 | USD 19.69 Billion |
| Growth Rate | CAGR of 9.10% |
| Number of Pages | 215 |
| Key Companies Covered | Laboratory Corporation of America Holdings (Labcorp), IQVIA Inc., Medpace Holdings Inc., Syneos Health, PAREXEL International, Eurofins Scientific SE, CROMSOURCE, Qserve Group, ICON plc, Fortrea Holdings Inc., Charles River Laboratories, Promedica International, WuXi AppTec, Avania, NAMSA, and others. |
| Segments Covered | By Device Type, By Operation Scale, By Target Indication, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Medical Device CRO Market: Segmentation
The global medical device CRO market is segmented based on device type, operation scale, target indication, and region.
Based on device type, the global market segments are handheld diagnostic devices, medtech devices, diagnostic devices, and others. In 2024, the highest growth was listed in the medtech devices segment. It includes a wide range of commonly used medical devices such as wearables and surgical robots. Furthermore, increased demand for advanced medical devices will accelerate segmental revenue and generate a CAGR of around 7%.
Based on operation scale, the global medical device CRO industry is divided into clinical and preclinical.
Based on the target indication, the global market divisions are metabolic disorders, pain disorders, oncological disorders, cardiovascular disorders, psychological disorders, neurological disorders, and others. In 2024, oncological disorders were the leading revenue generator. The growing prevalence of cancer worldwide and rising fatalities caused by the disease are assisting the segmental revenue. It is estimated that nearly 10 million people die every year due to cancer.
Medical Device CRO Market: Regional Analysis
North America to be led by the US during the forecast period
The global medical device CRO market is expected to be led by North America during the projection period. The US will emerge as the highest regional market revenue generator. The presence of influential players in the country is one of the major growth propellers for the region. For instance, leading medical device CRO brands such as Syneos Health, Medpace, and PRA Health Sciences are headquartered in the US. Furthermore, North America’s economy is driven by the rising interest in developing modern and cutting-edge medical devices for patient care.
Europe is expected to emerge as the second-highest revenue generator in the medical device CRO industry. Germany, Ireland, and the UK will help fuel regional market expansion. In October 2024, Avania, a Netherlands-based medical technology contract research organization, announced the acquisition of Anagram, a Spain-based company. The latter is a prominent MedTech CRO in the Iberian Peninsula. Moreover, the growing prevalence of serious medical conditions in Europe will further help the region thrive during the forecast period.
Asia-Pacific region is another flourishing market, with China and India leading the regional landscape. The ongoing advancements in medical device technology in the region and regulatory complexities will promote regional market expansion. Moreover, increased regional government support to encourage the medical device CRO sector will play a crucial role in the final revenue generated by Asia-Pacific.
Medical Device CRO Market: Competitive Analysis
The global medical device CRO market is led by players like:
- Laboratory Corporation of America Holdings (Labcorp)
- IQVIA Inc.
- Medpace Holdings Inc.
- Syneos Health
- PAREXEL International
- Eurofins Scientific SE
- CROMSOURCE
- Qserve Group
- ICON plc
- Fortrea Holdings Inc.
- Charles River Laboratories
- Promedica International
- WuXi AppTec
- Avania
- NAMSA
The global medical device CRO market is segmented as follows:
By Device Type
- Handheld Diagnostic Device
- MedTech Devices
- Diagnostic Devices
- Others
By Operation Scale
- Clinical
- Preclinical
By Target Indication
- Metabolic Disorders
- Pain Disorders
- Oncological Disorders
- Cardiovascular Disorders
- Psychological Disorders
- Neurological Disorders
- Others
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Medical device contract research organizations (CRO) are strategic partners to medical device companies.
The global medical device CRO market is expected to be driven by the changing landscape across the globe concerning the regulatory framework for medical devices.
According to study, the global medical device CRO market size was worth around USD 8.24 billion in 2024 and is predicted to grow to around USD 19.69 billion by 2034.
The CAGR value of the medical device CRO market is expected to be around 9.10% during 2025-2034.
The global medical device CRO market is expected to be led by North America during the projection period.
The global medical device CRO market is led by players like Laboratory Corporation of America Holdings (Labcorp), IQVIA Inc., Medpace Holdings, Inc., Syneos Health, PAREXEL International, Eurofins Scientific SE, CROMSOURCE, Qserve Group, ICON plc, Fortrea Holdings Inc., Charles River Laboratories, Promedica International, WuXi AppTec, Avania, and NAMSA.
The report explores crucial aspects of the medical device CRO market, including a detailed discussion of existing growth factors and restraints, while also browsing future growth opportunities and challenges that impact the market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed