Global Blood Glucose Monitoring System Market Size, Share, Growth Analysis Report - Forecast 2034

Blood Glucose Monitoring System Market By Product (Self-Monitoring and Continuous Glucose Monitoring Devices), By Testing Site (Fingertip Testing and Alternate Site Testing), Indication (Type-I Diabetes, Type-II Diabetes, and Others), End-User (Hospitals, Clinics, and Homecare), and By Region: Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 14.55 Billion | USD 32.45 Billion | 8.35% | 2024 |
Blood Glucose Monitoring System Market: Industry Perspective
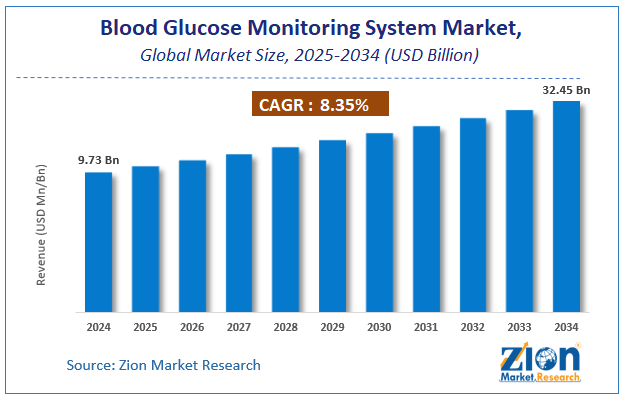
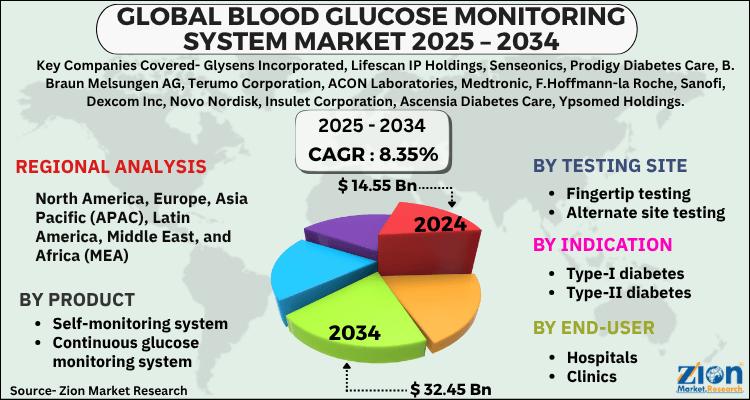
The global blood glucose monitoring system market size was worth around USD 14.55 Billion in 2024 and is predicted to grow to around USD 32.45 Billion by 2034 with a compound annual growth rate (CAGR) of roughly 8.35% between 2025 and 2034. The report analyzes the global blood glucose monitoring system market's drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the blood glucose monitoring system industry.
Blood glucose monitoring devices are used to detect the glucose level in the blood. Glucose is a type of sugar present at a certain level in a human body. When this glucose level drops, a person suffers from diabetes. Blood glucose monitoring devices are used to monitor the glucose levels of diabetic patients.
Blood Glucose Monitoring System Market: Overview
A blood glucose monitoring system also known as BGM is an effective tool used for monitoring blood sugar levels, especially for diabetic patients. Blood glucose monitoring systems have helped patients become self-reliant and take control of their medical health. BGM systems track high or low glucose levels and encourage patients to take relevant medication or treatment.
BGM systems work by understanding the resistance to an electric current sent through a blood-stained test strip. Or by inserting a sensor under the patient's skin that reads the glucose level between cell fluids. The type of blood glucose monitoring system to be used depends on the requirements of the patient. Multiple options for BGM devices are present in the market catering to different medical needs. The global blood glucose monitoring system is witnessing a surge in its global market value and is projected to touch milestones during the forecast period.
Key Insights
- As per the analysis shared by our research analyst, the global blood glucose monitoring system market is estimated to grow annually at a CAGR of around 8.35% over the forecast period (2025-2034).
- Regarding revenue, the global blood glucose monitoring system market size was valued at around USD 14.55 Billion in 2024 and is projected to reach USD 32.45 Billion by 2034.
- The blood glucose monitoring system market is projected to grow at a significant rate due to rising prevalence of diabetes, increasing adoption of continuous glucose monitors (CGMs), technological advancements, and growing demand for non-invasive monitoring solutions.
- Based on Product, the Self-Monitoring segment is expected to lead the global market.
- On the basis of Testing Site, the Fingertip Testing segment is growing at a high rate and will continue to dominate the global market.
- Based on the Indication, the Type-I Diabetes segment is projected to swipe the largest market share.
- By End-User, the Hospitals segment is expected to dominate the global market.
- Based on region, North America is predicted to dominate the global market during the forecast period.
Blood Glucose Monitoring System Market: Growth Drivers
The increasing population of diabetic patients drives the global market growth
The number of patients worldwide suffering from type 1 and type 2 diabetes has grown exponentially over the last couple of years. The changing stationary lifestyle, poor eating habits, and genetic disorders are the leading cause of growth in diabetic patients. This rising insulin dependency is expected to propel the global blood glucose monitoring system market toward growth in the forecast period. In 2021, International Diabetes Federation published a report, the findings of which stated that around 537M of the population in countries like India, Mexico, and China suffer from diabetes.
The presence of multiple manufacturers of blood glucose monitoring systems offering technically advanced devices at favorable prices is anticipated to contribute to the global market growth during the forecast period. Developed economies having state-of-the-art medical facilities are expected to fuel global market growth.
Blood Glucose Monitoring System Market: Restraints
Lack of awareness along with the high cost of devices in emerging economies restrain the global market
A fair share of the population in growing economies is unaware of diabetes and its management. Furthermore, developing economies lack the necessary infrastructure for treatment facilities to manage chronic diseases like diabetes. The lack of awareness and absence of advanced medical facilities is expected to hamper the blood glucose monitoring system market during the forecast period.
Emerging economies are also lagging in the necessary funding required for R&D which restricts the global market growth. SMBG and CGM are invasive techniques. Patients’ hesitancy while using invasive methods for tracking diabetes is projected to restrict global market growth.
Blood Glucose Monitoring System Market: Opportunities
Explosive amount of untapped market to provide growth opportunities in the global market
As per IDF's 2021 report, half of the total diabetic patients have remained undiagnosed in Africa. Around 56% of the Asia-Pacific population comprises undiagnosed patients. This provides a massive opportunity for global market leaders to tap into.
The country's governments have been taking proactive actions, conducting programs to educate the masses about diabetes and ways to control it. The global blood glucose monitoring system market can bank upon these initiatives to provide growth opportunities in the forecast period.
Blood Glucose Monitoring System Market: Challenges
Lack of funding presents a challenge for the global market growth
Lack of funding for technical advancement in the field of blood glucose monitoring systems poses a major challenge in the global market during the forecast period. Developed countries have shown more acceptance towards BGM devices owing to better awareness and technically rich devices However, developing economies need more investment to penetrate every corner of the target global market.
For developing nations, the prices of BGM devices can be relatively higher than in developed nations. The maintenance and supporting accessories increase the overall cost of the device making it less cost-effective for patients in these territories. This is a major challenge for the global market to tackle.
Diabetes is one of the few medical conditions which are not reimbursed by healthcare agencies. The absence of reimbursement, particularly in SMBG, challenges the upward global market growth. Since the disease is not reimbursed, a potential number of patients fail to get themselves tested for diabetes and a huge chunk of the global market remains in isolation.
Blood Glucose Monitoring System Market: Segmentation
The global blood glucose monitoring system is segmented based on product, testing site, indication, end-user, and region.
Based on product, the global market is segmented between self-monitoring and continuous glucose monitoring devices. SBGM is expected to maintain the highest share in the global market owing to its cost-effectiveness and hassle-free usage.
Based on testing sites, the global market is segmented between fingertip testing and alternate site testing. Fingertip testing is anticipated to cover the market share more than alternate site testing during the forecast period.
Based on indication, the global market is segmented between type-I diabetes, type-II diabetes, and others. As of 2021, the global market share was dominated by type-II diabetic patients because of more patients suffering from type-II diabetic conditions.
Based on end-user, the global market is segmented by hospitals, clinics, and home care. In 2021, the hospital segment marked the highest revenue and is anticipated to lead the global market during the forecast part owing to heavy investments in the hospital segment.
Blood Glucose Monitoring System Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Blood Glucose Monitoring System Market |
| Market Size in 2024 | USD 14.55 Billion |
| Market Forecast in 2034 | USD 32.45 Billion |
| Growth Rate | CAGR of 8.35% |
| Number of Pages | 205 |
| Key Companies Covered | Glysens Incorporated, Lifescan IP Holdings, Senseonics, Prodigy Diabetes Care, B. Braun Melsungen AG, Terumo Corporation, ACON Laboratories, Medtronic, F.Hoffmann-la Roche, Sanofi, Dexcom Inc, Novo Nordisk, Insulet Corporation, Ascensia Diabetes Care, Ypsomed Holdings, Nova Biomedical, and others. |
| Segments Covered | By Product, By Testing Site, By Indication, By End-User, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, The Middle East and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2020 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Blood Glucose Monitoring System Market: Regional landscape
The global market is likely to be led by North America during the forecast period
North America was attributed with the highest global blood glucose monitoring system market in 2021 owing to a 35.5% share of the global revenue. Prevalence of obesity in addition to the high standard of medical assistance provided to patients, technically advanced equipment is expected to assist North America in staying the highest revenue contributor during the forecast period. Europe shares a significant amount of global market share because of the awareness and proactive approach of its population in diagnosing and managing diabetes. Europe is expected to continue its growth streak because of the intense investment in healthcare facilities.
Asia Pacific is anticipated to register a higher CAGR during the forecast period owing to growing awareness & acceptance of BGM systems, particularly in India & China. The increase in investments in medical facilities is also a major cause of the anticipated growth. The slow but steady growth in the spending capability of the general population is another factor expected to contribute to the global market. The Middle East, Latin America & Africa are expected to show slow growth majorly because of a lack of awareness amongst the population about proper diabetes management. The number of undiagnosed patients in these regions still remains high causing a lagging growth graph in the global market. Below-par standard of living is also one of the causes of registering slower CAGR.
Recent Developments:
- In January 2022, a non-invasive blood glucose testing prototype was invented by Scanbo, that provides blood glucose results in 60 secs. This invention will help cater to diabetic patients who are less comfortable with invasive blood glucose testing methods.
- In March 2021, Omron Healthcare Inc. announced its expansion plans in India in the year 2021. Omron is a health device manufacturing company and its intention is to increase BGM device retails by 35 to 40%, in India.
Blood Glucose Monitoring System Market: Competitive Analysis
The report provides a company market share analysis to give a broader overview of the key market players. In addition, the report also covers key strategic developments of the market, including acquisitions & mergers, new product launches, agreements, partnerships, collaborations & joint ventures, research & development, and regional expansion of major participants involved in the blood glucose monitoring system market on a global and regional basis.
The global blood glucose monitoring system market is dominated by players like:
- Glysens Incorporated
- Lifescan IP Holdings
- Senseonics
- Prodigy Diabetes Care
- B. Braun Melsungen AG
- Terumo Corporation
- ACON Laboratories, Medtronic
- F.Hoffmann-la Roche
- Sanofi
- Dexcom Inc
- Novo Nordisk
- Insulet Corporation
- Ascensia Diabetes Care
- Ypsomed Holdings
- Nova Biomedical.
The global blood glucose monitoring system market is segmented as follows:
By Product:
- Self-monitoring system
- Continuous glucose monitoring system
By Testing Site:
- Fingertip testing
- Alternate site testing
By Indication:
- Type-I diabetes
- Type-II diabetes
- Others
By End-User
- Hospitals
- Clinics
- Homecare
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of The Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
A blood glucose monitoring system also known as BGM is an effective tool used for monitoring blood sugar levels, especially for diabetic patients.
The global blood glucose monitoring system market is expected to grow due to increasing adoption of continuous glucose monitoring (CGM) devices, rising prevalence of diabetes, technological advancements, and growing awareness of diabetes management.
According to a study, the global blood glucose monitoring system market size was worth around USD 14.55 Billion in 2024 and is expected to reach USD 32.45 Billion by 2034.
The global blood glucose monitoring system market is expected to grow at a CAGR of 8.35% during the forecast period.
North America is expected to dominate the blood glucose monitoring system market over the forecast period.
Leading players in the global blood glucose monitoring system market include Glysens Incorporated, Lifescan IP Holdings, Senseonics, Prodigy Diabetes Care, B. Braun Melsungen AG, Terumo Corporation, ACON Laboratories, Medtronic, F.Hoffmann-la Roche, Sanofi, Dexcom Inc, Novo Nordisk, Insulet Corporation, Ascensia Diabetes Care, Ypsomed Holdings, Nova Biomedical, among others.
The report explores crucial aspects of the blood glucose monitoring system market, including a detailed discussion of existing growth factors and restraints, while also examining future growth opportunities and challenges that impact the market.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed