Global CAR T-Cell Therapy Market Size, Share, Growth Analysis Report - Forecast 2034

CAR T-Cell Therapy Market By Product (Abecma (idecabtagene vicleucel), Breyanzi (lisocabtagene maraleucel), Carvykti (ciltacabtagene autoleucel), Kymriah (tisagenlecleucel), Tecartus (brexucabtagene autoleucel), Yescarta (axicabtagene ciloleucel), Others), By Disease Indication (Leukemia, Lymphoma, Multiple Myeloma, Others), By End-Use (Hospitals, Cancer Treatment Centers), and By Region: Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 609.41 Million | USD 6594.52 Million | 26.89% | 2024 |
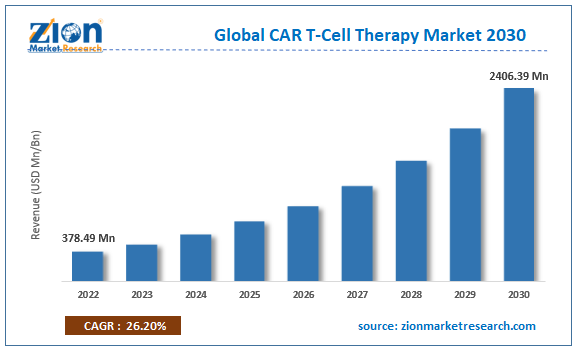
Global CAR T-Cell Therapy Market: Industry Perspective
The global car t-cell therapy market size was worth around USD 609.41 Million in 2024 and is predicted to grow to around USD 6594.52 Million by 2034 with a compound annual growth rate (CAGR) of roughly 26.89% between 2025 and 2034. The report analyzes the global car t-cell therapy market's drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the car t-cell therapy industry.
Global CAR T-Cell Therapy Market: Overview
CAR T-cell therapy is a kind of cancer immunotherapy that makes use of genetically modified immune cells referred to as T-cells for locating cancer tissues and destroying them. In addition to this, there are three steps involved in the CAR T-cell therapy that include collection of T-cells, infusing of CAR-T cells in the human body, and T-cell engineering. For the record, CAR T-cell therapy is an FDA-approved treatment that can be utilized for treating hematological malignancies such as multiple myeloma, leukemia, and lymphoma.
Global CAR T-Cell Therapy Market: Growth Factors
The rise in the occurrence of cancer and demand for apt modes of treating the disease is set to impel the expansion of the global CAR T-cell therapy market. Additionally, favorable compensation policies and an increase in awareness about this treatment mode will prompt global market trends. Moreover, less time is required by CAR T-cell therapy for treating the cancer disease and this will catapult the global market trends. Reportedly, clinical tests in blood cancers have demonstrated that patients in whom cancer recurred after various modes of treatments, when treated with CAR T-cell therapy achieved remissions which lasted for many years. Furthermore, subjects treated with CAR T-cell therapy also benefitted from curative cancer therapy such as stem cell transplants. All these aforementioned aspects are likely to steer the growth of the global market in the years to come. Moreover, six CAR T-cell therapies have been approved by the U.S. FDA since 2017. All these therapies are approved for treating blood cancers such as a few types of leukemia, lymphomas, and multiple myeloma.
Restraints:
Side effects of CAR T-cell therapy include headache, high fever, nausea, diarrhea, joint pain, vomiting, and loss of balance. In addition to this, other side effects of the therapy include allergic reactions, bleeding, fatigue, dizziness, low blood pressure, and difficulty in breathing. All these above-mentioned aspects can impede the growth of the CAR T-cell therapy industry across the globe.
Opportunities:
Strategic partnerships & new product launches can create new opportunities for growth in the global market
Launching new product will open new growth avenues for the global CAR T-cell therapy market. Furthermore, mergers & acquisitions, and partnerships will contribute lucratively towards the expansion of the global market in the upcoming years. For instance, in the first half of 2018, Gilead Sciences Inc., a biotech firm based in the U.S., joined hands with Sangamo Therapeutics Inc., a U.S.-based biotech company, for bringing improvements in gene editing techniques for treating cancer.
Challenges:
Demand for proficient manpower for executing CAR T-cell therapy can prove as a challenging factor in the growth of the global industry
The need for an efficient workforce for developing and implementing CAR T-cell treatments can prove to be a huge challenge for the expansion of the global CAR T-cell therapy industry. Other challenges faced by healthcare service providers include antigen escape, restricted tumor infiltration, and limited trafficking.
Key Insights
- As per the analysis shared by our research analyst, the global car t-cell therapy market is estimated to grow annually at a CAGR of around 26.89% over the forecast period (2025-2034).
- Regarding revenue, the global car t-cell therapy market size was valued at around USD 609.41 Million in 2024 and is projected to reach USD 6594.52 Million by 2034.
- The car t-cell therapy market is projected to grow at a significant rate due to rising incidence of cancer, particularly hematological malignancies, increasing investments and funding in CAR T-cell therapy research and development, and continuous advancements in genetic engineering and personalized medicine.
- Based on Product, the Abecma (idecabtagene vicleucel) segment is expected to lead the global market.
- On the basis of Disease Indication, the Leukemia segment is growing at a high rate and will continue to dominate the global market.
- Based on the End-Use, the Hospitals segment is projected to swipe the largest market share.
- Based on region, North America is predicted to dominate the global market during the forecast period.
Global CAR T-Cell Therapy Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | CAR T-Cell Therapy Market |
| Market Size in 2024 | USD 609.41 Million |
| Market Forecast in 2034 | USD 6594.52 Million |
| Growth Rate | CAGR of 26.89% |
| Number of Pages | 222 |
| Key Companies Covered | Bristol-Myers Squibb Company, Novartis AG, Gilead Sciences, Inc., Johnson & Johnson Services, Inc., JW Therapeutics (Shanghai) Co., Ltd., bluebird bio, Inc., Merck & Co., Inc., Sangamo Therapeutics, Sorrento Therapeutics, Inc., GSK plc., and others. |
| Segments Covered | By Product, By Disease Indication, By End-Use, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, The Middle East and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2020 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Global CAR T-Cell Therapy Market: Segmentation Analysis
The global car t-cell therapy market is segmented based on Product, Disease Indication, End-Use, and region. All the segments have been analyzed based on present and future trends and the market is estimated from 2025 to 2034.
Based on Product, the global car t-cell therapy market is divided into Abecma (idecabtagene vicleucel), Breyanzi (lisocabtagene maraleucel), Carvykti (ciltacabtagene autoleucel), Kymriah (tisagenlecleucel), Tecartus (brexucabtagene autoleucel), Yescarta (axicabtagene ciloleucel), Others.
On the basis of Disease Indication, the global car t-cell therapy market is bifurcated into Leukemia, Lymphoma, Multiple Myeloma, Others.
By End-Use, the global car t-cell therapy market is split into Hospitals, Cancer Treatment Centers.
The Regional, this segment includes the current and forecast demand for North America, Europe, Asia Pacific, Latin America,and the Middle East and Africa.
Global CAR T-Cell Therapy Market: Regional Insights
The CAR T-cell therapy market shows significant regional variation, with North America leading due to advanced healthcare infrastructure, strong research and development activities, and early regulatory approvals. The United States dominates the region, supported by high investment in immunotherapy and a strong presence of key biotechnology firms. Europe follows closely, driven by increasing clinical trials, rising adoption of advanced cancer treatments, and government support for personalized medicine. The Asia-Pacific region is witnessing rapid growth, especially in China and Japan, fueled by expanding healthcare access, growing awareness of cell-based therapies, and increasing investment in biopharmaceutical research. Meanwhile, regions like Latin America and the Middle East are gradually emerging as markets, supported by improved healthcare systems and growing demand for innovative cancer treatments.
Global CAR T-Cell Therapy Market: Competitive Analysis
The report provides a company market share analysis to give a broader overview of the key market players. In addition, the report also covers key strategic developments of the market, including acquisitions & mergers, new product launches, agreements, partnerships, collaborations & joint ventures, research & development, and regional expansion of major participants involved in the car t-cell therapy market on a global and regional basis.
The global car t-cell therapy market is dominated by players like:
- Bristol-Myers Squibb Company
- Novartis AG
- Gilead Sciences Inc.
- Johnson & Johnson Services Inc.
- JW Therapeutics (Shanghai) Co. Ltd.
- bluebird bio Inc.
- Merck & Co. Inc.
- Sangamo Therapeutics
- Sorrento Therapeutics Inc.
- GSK plc.
Global CAR T-Cell Therapy Market: Segmentation Analysis
The global car t-cell therapy market is segmented as follows;
By Product
- Abecma (idecabtagene vicleucel)
- Breyanzi (lisocabtagene maraleucel)
- Carvykti (ciltacabtagene autoleucel)
- Kymriah (tisagenlecleucel)
- Tecartus (brexucabtagene autoleucel)
- Yescarta (axicabtagene ciloleucel)
- Others
By Disease Indication
- Leukemia
- Lymphoma
- Multiple Myeloma
- Others
By End-Use
- Hospitals
- Cancer Treatment Centers
Global CAR T-Cell Therapy Market: Regional Segment Analysis
- North America
- The U.S.
- Canada
- Mexico
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of Asia Pacific
- The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
Table Of Content
Methodology
FrequentlyAsked Questions
CAR T-cell therapy is a kind of cancer immunotherapy that makes use of genetically modified immune cells referred to as T-cells for locating cancer tissues and destroying them. In addition to this, there are three steps involved in the CAR T-cell therapy that include collection of T-cells, infusing of CAR-T cells in the human body, and T-cell engineering. For the record, CAR T-cell therapy is an FDA-approved treatment that can be utilized for treating hematological malignancies such as multiple myeloma, leukemia, and lymphoma.
The global car t-cell therapy market is expected to grow due to increasing prevalence of cancer, advancements in immunotherapy, rising R&D investments, and growing regulatory approvals for innovative treatments.
According to a study, the global car t-cell therapy market size was worth around USD 609.41 Million in 2024 and is expected to reach USD 6594.52 Million by 2034.
The global car t-cell therapy market is expected to grow at a CAGR of 26.89% during the forecast period.
North America is expected to dominate the car t-cell therapy market over the forecast period.
Leading players in the global car t-cell therapy market include Bristol-Myers Squibb Company, Novartis AG, Gilead Sciences, Inc., Johnson & Johnson Services, Inc., JW Therapeutics (Shanghai) Co., Ltd., bluebird bio, Inc., Merck & Co., Inc., Sangamo Therapeutics, Sorrento Therapeutics, Inc., GSK plc., among others.
The report explores crucial aspects of the car t-cell therapy market, including a detailed discussion of existing growth factors and restraints, while also examining future growth opportunities and challenges that impact the market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed