Cardiac Safety Services Market Size, Share Report, Analysis, Trends, Growth, 2032

Cardiac Safety Services Market by Type (Standalone and Integrated Services); Service Type (Cardiovascular Imaging, Blood Pressure Measurement, Thorough QT Studies, ECG/Holter Measurement and Other Services); by End User (Contract Research Organizations (CROs), Biopharmaceutical and Pharmaceutical Companies and Other End Users) and by Region: Global Industry Perspective, Comprehensive Analysis, and Forecast, 2024 - 2032-
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
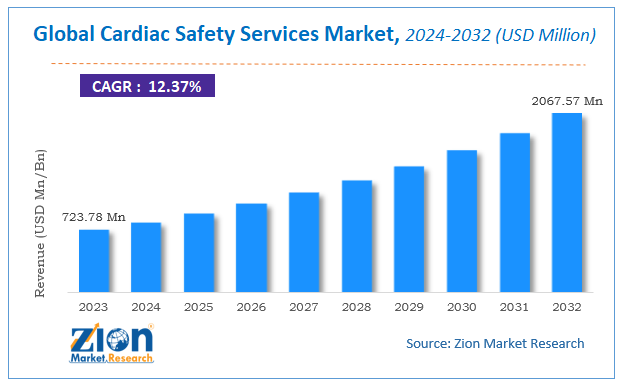
| USD 723.78 Million | USD 2067.57 Million | 12.37% | 2023 |
Cardiac Safety Services Market Insights
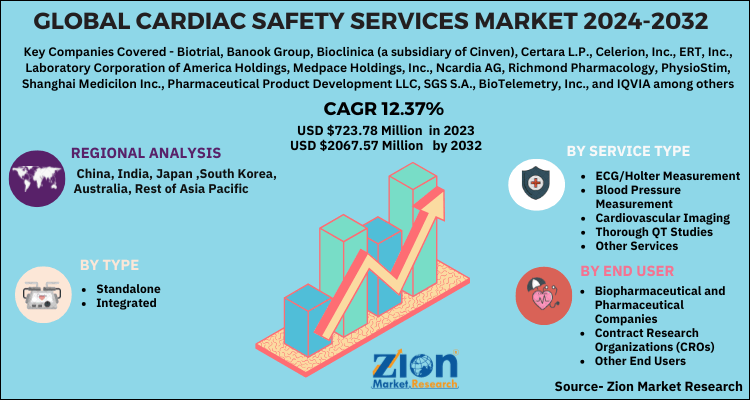
According to Zion Market Research, the global Cardiac Safety Services Market was worth USD 723.78 Million in 2023. The market is forecast to reach USD 2067.57 Million by 2032, growing at a compound annual growth rate (CAGR) of 12.37% during the forecast period 2024-2032.
The report offers a comprehensive analysis of the market, highlighting the factors that will determine growth, potential challenges, and opportunities that could emerge in the Cardiac Safety Services industry over the next decade.
The report analyzes the cardiac safety services market’s drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the cardiac safety services market.
The report covers forecasts and analyses for the cardiac safety services market on a global and regional level. The study provides historic data from 2018 to 2023 along with a forecast from 2024-2032 based on revenue (USD Million). The study includes drivers and restraints for the cardiac safety services market along with the impact they have on the demand over the forecast period. Additionally, the report includes the study of opportunities available in the cardiac safety services market on a global as well as regional level.
In order to give the users of this report a comprehensive view of the cardiac safety services market we have included a competitive landscape and analysis of Porter’s Five Forces model for the market. The study encompasses a market attractiveness analysis, wherein all segments are benchmarked based on their market size, growth rate, and general attractiveness.
Global Cardiac Safety Services Market: Overview
The report provides a company market share analysis in order to give a broader overview of the key players in the cardiac safety services market. In addition, the report also covers key strategic developments of the market including acquisitions & mergers, new type launches, agreements, partnerships, collaborations & joint ventures, research & development, and regional expansion of major participants involved in the cardiac safety services market on a global and regional basis.
The study provides a crucial view of cardiac safety services by segmenting the market based on type, service type, end user, and region. All the segments of the cardiac safety services market have been analyzed based on present and future trends and the market is estimated from 2023 to 2032.
Global Cardiac Safety Services Market: Growth Factors
Increasing R&D expenditures in the biopharmaceutical and pharmaceutical industries, an uptick in the number of clinical trials, and a growing preference for outsourcing R&D operations are some of the reasons that are driving the global demand for the cardiac safety services market. Some of the other aspects that are likely to offer up new growth prospects for market players operating in the cardiac safety services market include the introduction of new methodologies and technologies, the expanding use of biosimilars, and the increasing market growth of biologics and biosimilars.
On the other hand, the high cost of safety evaluation may function as factors that act as restraints for the growth of the market in the coming years. In the future years, there are a number of reasons that are anticipated to provide new growth avenues for market players. These aspects include the introduction of new procedures and technologies, as well as an extensive biologics and biosimilar medication pipeline.
Cardiac Safety Services Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Cardiac Safety Services Market |
| Market Size in 2023 | USD 723.78 Million |
| Market Forecast in 2032 | USD 2067.57 Million |
| Growth Rate | CAGR of 12.37% |
| Number of Pages | 110 |
| Key Companies Covered | Biotrial, Banook Group, Bioclinica (a subsidiary of Cinven), Certara L.P., Celerion, Inc., ERT, Inc., Laboratory Corporation of America Holdings, Medpace Holdings, Inc., Ncardia AG, Richmond Pharmacology, PhysioStim, Shanghai Medicilon Inc., Pharmaceutical Product Development LLC, SGS S.A., BioTelemetry, Inc., and IQVIA among others |
| Segments Covered | By Indication, By Drug Class, By Route Of Administration, By Distribution Channel And By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Global Cardiac Safety Services Market: Segmentation
Based on type, the global cardiac safety services market is bifurcated into standalone and integrated. Based on service type, the global cardiac safety services market is categorized into cardiovascular imaging, blood pressure measurement, thorough QT studies, ECG/Holter measurement, and other services. Based on the end-user market is segmented into contract research organizations (CROs), biopharmaceutical and pharmaceutical companies, and other end users.
The regional segment includes North America, Latin America, Asia Pacific, Europe, and the Middle East and Africa.
Global Cardiac Safety Services Market: Competitive Players
Some major players in the cardiac safety services market are
- Biotrial
- Banook Group
- Bioclinica (a subsidiary of Cinven)
- Certara L.P.
- Celerion. Inc.
- ERT. Inc.
- Laboratory Corporation of America Holdings
- Medpace Holdings. Inc.
- Ncardia AG
- Richmond Pharmacology
- PhysioStim
- Shanghai Medicilon Inc.
- Pharmaceutical Product Development LLC
- SGS S.A.
- BioTelemetry. Inc.
- IQVIA
- among others.
The report segment of global cardiac safety services market as follows:
Global Cardiac Safety Services Market: Type
- Standalone
- Integrated
Global Cardiac Safety Services Market: By Service Type
- ECG/Holter Measurement
- Blood Pressure Measurement
- Cardiovascular Imaging
- Thorough QT Studies
- Other Services
Global Cardiac Safety Services Market: By End User
- Biopharmaceutical and Pharmaceutical Companies
- Contract Research Organizations (CROs)
- Other End Users
Global Cardiac Safety Services Market: By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Cardiovascular devices are types of medical equipment that can diagnose, treat, or manage a variety of illnesses that are related to the cardiovascular system (the heart and blood arteries). These tools contribute significantly to the advancement of contemporary medical practice by facilitating the monitoring, prevention, and treatment of cardiovascular illnesses. They comprise a wide variety of technology and techniques that help in restoring blood flow, evaluating heart function, and bolstering overall cardiovascular health.
The global demand for the cardiac safety services market is driven by factors such as increasing R&D expenditure in the biopharmaceutical and pharmaceutical industry, the surge in a number of clinical trials, and increasing preference for outsourcing of R&D activities.
global Cardiac Safety Services Market was worth USD 723.78 Million in 2023. The market is forecast to reach USD 2067.57 Million by 2032, growing at a compound annual growth rate (CAGR) of 12.37% during the forecast period 2024-2032
North America held the largest revenue share of the global cardiac safety services market in 2018. This large share is attributed to the strong presence of key biopharmaceutical and pharmaceutical players in this region.
Major players in cardiac safety services market are Biotrial, Banook Group, Bioclinica (a subsidiary of Cinven), Certara L.P., Celerion, Inc., ERT, Inc., Laboratory Corporation of America Holdings, Medpace Holdings, Inc., Ncardia AG, Richmond Pharmacology, PhysioStim, Shanghai Medicilon Inc., Pharmaceutical Product Development LLC, SGS S.A., BioTelemetry, Inc., and IQVIA among others.
Choose License Type
List of Contents
Market InsightsGlobal Cardiac Safety ServicesMarket:OverviewGlobal Cardiac Safety ServicesGrowth FactorsReport ScopeGlobal Cardiac Safety ServicesMarket:SegmentationGlobal Cardiac Safety ServicesCompetitive PlayersThe report segment of global cardiac safety services market as follows:RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed