Clinical Trial Imaging Market Size, Share, Trends, 2032

Clinical Trial Imaging Market By Services (Project and Data Management, Clinical Trial Design and Consultation, Reading and Analytical, Operational Imaging and Others), By Modality (CT, MRI, PET, Ultrasound, X-Ray and Others), By End Use (Biotechnology and Pharmaceutical companies, Medical Devices Manufacturers, Contract Research Organizations (CROs), Academic and Government Research Institutes and Others): Global Industry Perspective, Comprehensive Analysis and Forecast, 2024 - 2032-
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 1287.5 Million | USD 2628.37 Million | 7.4% | 2023 |
Clinical Trial Imaging Market Insights
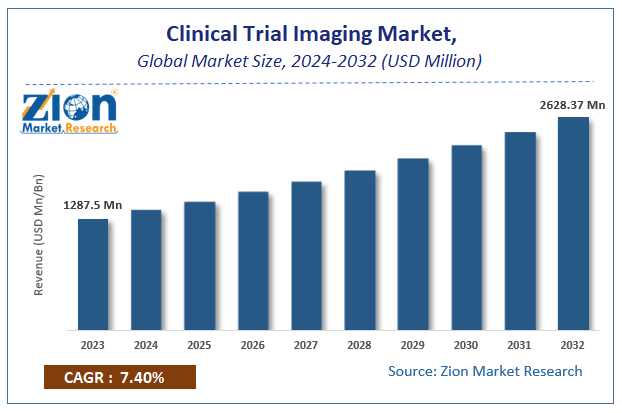
Zion Market Research has published a report on the global Clinical Trial Imaging Market, estimating its value at USD 1287.5 Million in 2023, with projections indicating that it will reach USD 2628.37 Million by 2032. The market is expected to expand at a compound annual growth rate (CAGR) of 7.4% over the forecast period 2024-2032. The report explores the factors fueling market growth, the hitches that could hamper this expansion, and the opportunities that may arise in the Clinical Trial Imaging Market industry. Additionally, it offers a detailed analysis of how these elements will affect market demand dynamics and market performance throughout the forecast period.
Global Clinical Trial Imaging Market Overview
Imaging has traditionally played 3 roles that relate to clinical trials: detection, characterization, and monitoring/assessment. Over the last two decades, the use of imaging in clinical trials has risen exponentially. In many trials, imaging data is increasingly being used to support the primary endpoint. An increase in the number of contract research organizations, in the geriatric population with chronic diseases, and a rise in R&D expenditure are some of the factors that would drive growth of the clinical trial imaging market.
Furthermore, the emergence of pharmaceutical and biotechnology sectors, as well as of small and portable equipments market is likely to give opportunities for the clinical trial imaging market to grow. The improvements and advancements in medical imaging technology and their increasing use throughout the world are fuelling the demand for imaging technologies in clinical trials.
COVID-19 Impact Analysis
During the COVID-19 pandemic, the unprecedented demand for hospital services severely hampered the ability to respond to non-acute health requirements. Non-COVID-19-related research activity has had to be curtailed in order to relieve the load on health-care systems, especially imaging and laboratory services. To lower the danger of infection, measures to reduce hospital visits have been implemented.
Imaging, on the other hand, necessitates hospital visits and intimate contact with workers and equipment, both of which might spread disease. Unless cleaned, equipment used to image COVID-19 patients may retain virus on its surface for days. Imaging processing is significantly slowed and throughput is reduced due to the necessity for social distancing and disinfection equipment.
Clinical Trial Imaging Market: Growth Factors
Pharmaceutical companies have grown in popularity across the world as science and technology have advanced. As the number of pharmaceutical and biotechnology companies grow, it has become increasingly important for manufacturers to provide the finest possible treatment or therapy to end users, as the companies face more competition.
The rise in demand for clinical trial imaging is primarily due to the growing geriatric population and the rising prevalence of chronic diseases. Another factor driving the growth of the clinical trial imaging market is increasing research and development capabilities for drug discovery against a variety of ailments.
Services Segment Analysis Preview
This is attributable to the fact that imaging-based clinical trials often need data management and workflow integration involving several stakeholders. Operational expertise and trial workflow development, project tracking, converting scans into digital images, regulatory control & quality assurance, real-time trial status report, setup and management of MRI centers, data management, and reporting and issue resolution are some of the services offered. Few governments across the globe have authorized the use of cloud-based servers that protect all medical imaging information, including annotated and basic pictures, protects reports from natural disasters, and allows for faster and easier retrieval.
End Use Industry Segment Analysis Preview
This is attributable to the necessity to find innovative treatments and therapies to cure chronic diseases. As the number of biotechnology and pharmaceutical businesses grows, manufacturers must provide the greatest available medicine/drug to end-users to stay competitive. As biotechnology and pharmaceutical firms make more innovative drug discoveries, the need for clinical trial imaging will rise, further boosting the market.
Clinical Trial Imaging Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Clinical Trial Imaging Market |
| Market Size in 2023 | USD 1287.5 Million |
| Market Forecast in 2032 | USD 2628.37 Million |
| Growth Rate | CAGR of 7.4% |
| Number of Pages | 140 |
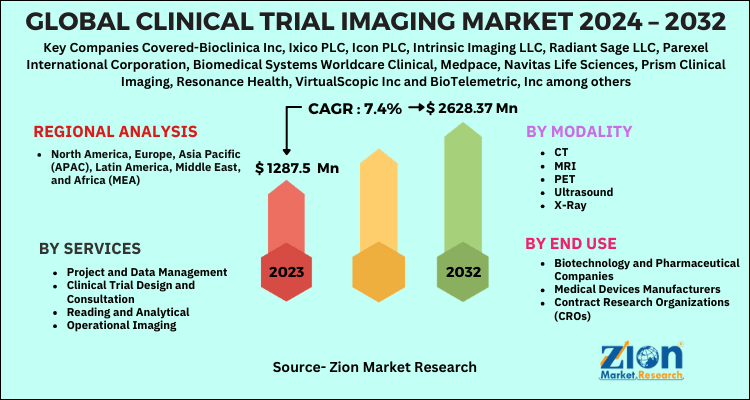
| Key Companies Covered | Bioclinica Inc, Ixico PLC, Icon PLC, Intrinsic Imaging LLC, Radiant Sage LLC, Parexel International Corporation, Biomedical Systems Worldcare Clinical, Medpace, Navitas Life Sciences, Prism Clinical Imaging, Resonance Health, VirtualScopic Inc and BioTelemetric, Inc among others |
| Segments Covered | By Services, By Modality, By End Use and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Clinical Trial Imaging Market: Regional Analysis Preview
This is attributable to the rising prevalence of chronic diseases and the growing geriatric population, the regional market is expected to rise. Aside from that, advancements in healthcare infrastructure have made it possible to diagnose and treat chronic diseases more efficiently. The US Food and Drug Administration (FDA) and other regulatory agencies have also played a key role in achieving trial registration objectives to date. Considering all of these factors, the regional market is expected to maintain its market position over the forecast period.
This can be attributed to the region's rapid population expansion and increased research and development activities. In addition, traditional and advanced devices and therapies are in great demand across the Asia Pacific region.
Clinical Trial Imaging Market Players & Competitive Landscape
Some of key players in clinical trial imaging market are
- Bioclinica Inc
- Ixico PLC
- Icon PLC
- Intrinsic Imaging LLC
- Radiant Sage LLC
- Parexel International Corporation
- Biomedical Systems Worldcare Clinical
- Medpace
- Navitas Life Sciences
- Prism Clinical Imaging
- Resonance Health
- VirtualScopic Inc
- BioTelemetric
The global clinical trial imaging market is segmented as follows:
By Services
- Project and Data Management
- Clinical Trial Design and Consultation
- Reading and Analytical
- Operational Imaging
- Others
By Modality
- CT
- MRI
- PET
- Ultrasound
- X-Ray
- Others
By End Use
- Biotechnology and Pharmaceutical Companies
- Medical Devices Manufacturers
- Contract Research Organizations (CROs)
- Academic and Government Research Institutes
- Others
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Zion Market Research has published a report on the global Clinical Trial Imaging Market, estimating its value at USD 1287.5 Million in 2023, with projections indicating that it will reach USD 2628.37 Million by 2032.
The market is expected to expand at a compound annual growth rate (CAGR) of 7.4% over the forecast period 2024-2032.
Some of the key factors driving the global clinical trial imaging market growth are increase in the number of contract research organizations, in the geriatric population with chronic diseases, and a rise in R&D expenditure, the emerging of pharmaceutical and biotechnology sectors, as well as of small and portable equipments market.
Asia Pacific region held a substantial share of the clinical trial imaging market in 2020. This can be attributed to the region's rapid population expansion and increased research and development activities. In addition, traditional and advanced devices and therapies are in great demand across the Asia Pacific region.
Some of the major companies operating in the clinical trial imaging market are Bioclinica Inc, Ixico PLC, Icon PLC, Intrinsic Imaging LLC, Radiant Sage LLC, Parexel International Corporation, Biomedical Systems Worldcare Clinical, Medpace, Navitas Life Sciences, Prism Clinical Imaging, Resonance Health, VirtualScopic Inc and BioTelemetric, Inc. among others.
Choose License Type
List of Contents
Market InsightsGlobal Market OverviewCOVID-19 Impact AnalysisGrowth FactorsServices Segment Analysis PreviewEnd Use Industry Segment Analysis PreviewReport ScopeRegional Analysis PreviewMarket Players Competitive LandscapeThe global clinical trial imaging market is segmented as follows:RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed