Medical Device Regulatory Affairs Market Size, Share, Growth and Value 2032

Medical Device Regulatory Affairs Market By Service (Legal Representation and Regulatory Consulting), By Type (Diagnostics and Therapeutics), And By Region- Global Industry Perspective, Comprehensive Analysis, and Forecast, 2024-2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 11.66 Billion | USD 20.37 Billion | 6.4% | 2023 |
Medical Device Regulatory Affairs Market Insights
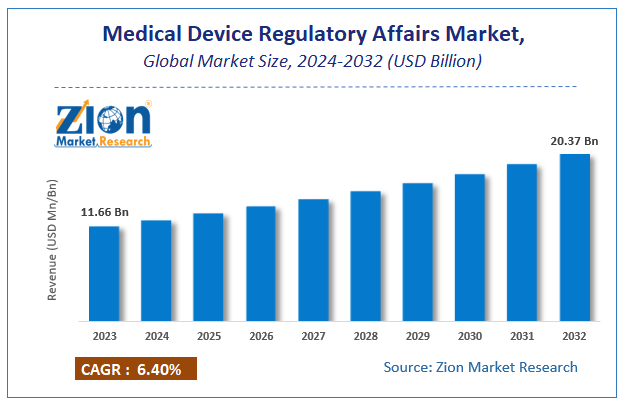
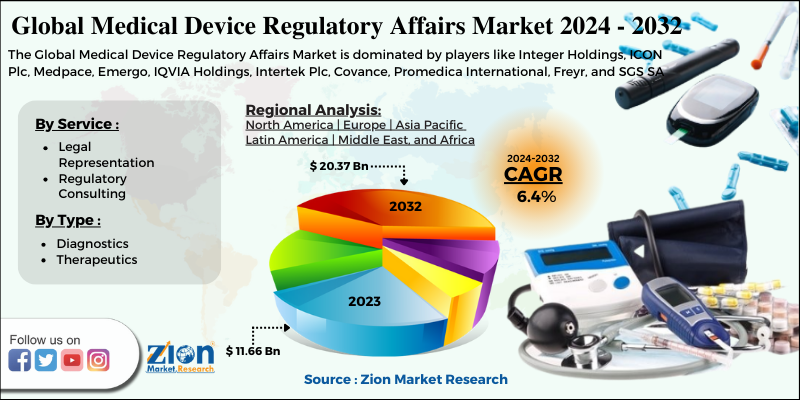
Zion Market Research has published a report on the global Medical Device Regulatory Affairs Market, estimating its value at USD 11.66 Billion in 2023, with projections indicating that it will reach USD 20.37 Billion by 2032. The market is expected to expand at a compound annual growth rate (CAGR) of 6.4% over the forecast period 2024-2032. The report explores the factors fueling market growth, the hitches that could hamper this expansion, and the opportunities that may arise in the Medical Device Regulatory Affairs Market industry. Additionally, it offers a detailed analysis of how these elements will affect market demand dynamics and market performance throughout the forecast period.
Medical Device Regulatory Affairs Market: Overview
The medical device sector plays a core role in the healthcare ecosystem by offering new solutions to enhance patient outcomes. Every firm needs to innovate by using iterative upgradation to current technology or developing new equipment that will provide proficient treatment for a particular ailment. With product developers trying to bring new ideas or solutions to reality, the regulatory affairs service providers play a key part by guiding the team on particular legislations for ensuring that the medical device can be marketed legally. Moreover, regulatory affairs solution providers also advise the product team as to how varied kinds of decisions impact the kind of regulatory submissions along with their deadlines.
Moreover, for products that are distributed globally, regulatory affairs coordinate with regional teams and ensure that the product possesses a global regulatory strategy. For maintaining the effectiveness of the medical device, it is necessary for regulatory affair professional for understanding the global regulatory needs of medical equipment along with assuring that the product development team takes into consideration the global needs of the product post testing & validation.
Medical Device Regulatory Affairs Market: Growth Drivers
Escalating demand for fast approval methods, shifting regulations, and swiftly expanding domains like diagnostics & therapeutics will steer the growth of the medical device regulatory affairs market in the years ahead. Apart from this, favorable government schemes and the growing complexity of medical equipment will embellish business space over the years to come. Growing cybersecurity threats and the fiscal impact of data violations on the sale of medical devices are making medical device producers accept strategies to safeguard their products, thereby driving market trends. Furthermore, the occurrence of various kinds of chronic disorders like heart ailments, diabetes, cancer, and respiratory issues will open new vistas of growth for the medical device regulatory affairs industry over the forthcoming years.
In addition to this, AI and machine learning tools help in extracting large amounts of data at the time of delivering healthcare services. With the massive demand for personal protective equipment kits and ventilators due to the large spread of the COVID-19 pandemic, the market for medical device regulatory affairs is anticipated to gain traction in the years ahead.
Medical Device Regulatory Affairs Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Medical Device Regulatory Affairs Market |
| Market Size in 2023 | USD 11.66 Billion |
| Market Forecast in 2032 | USD 20.37 Billion |
| Growth Rate | CAGR of 6.4% |
| Number of Pages | 130 |
| Key Companies Covered | Integer Holdings, ICON Plc, Medpace, Emergo, IQVIA Holdings, Intertek Plc, Covance, Promedica International, Freyr, and SGS SA |
| Segments Covered | By Service, By Type, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Medical Device Regulatory Affairs Market: Regional Landscape
The Asia Pacific To Make Notable Contributions Towards Overall Market Size By 2032
The expansion of the medical device regulatory affairs market in the Asia Pacific over the forecast timespan is due to massively evolved regulatory tools in countries such as Japan and Australia. Furthermore, supportive government policies for increasing FDAs and the need for increasing approval of new products will steer regional market growth. In addition to this, the necessity of relaxing laws related to procurement control will drive regional industry trends.
Medical Device Regulatory Affairs Market: Competitive Landscape
Key players profiled in the study include:
- Integer Holdings
- ICON Plc
- Medpace
- Emergo
- IQVIA Holdings
- Intertek Plc
- Covance
- Promedica International
- Freyr
- SGS SA
The global Medical Device Regulatory Affairs Market is segmented as follows:
By Service
- Legal Representation
- Regulatory Consulting
By Type
- Diagnostics
- Therapeutics
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of the Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Which key factors will influence the Medical Device Regulatory Affairs Market growth over 2024-2032?
Escalating demand for fast approval method, shifting regulations, and swiftly expanding domains like diagnostics & therapeutics will steer growth of medical device regulatory affairs market in the years ahead. Apart from this, favorable government schemes and growing complexity of medical equipment will embellish business space over the years to come. Growing cybersecurity threats and fiscal impact of data violations on sale of medical devices are making medical device producers accept strategies to safeguard their products, thereby driving market trends. Furthermore, occurrence of various kinds of chronic disorders like heart ailments, diabetes, cancer, and respiratory issues will open new vistas of growth for medical device regulatory affairs industry over the forthcoming years.
Zion Market Research has published a report on the global Medical Device Regulatory Affairs Market, estimating its value at USD 11.66 Billion in 2023, with projections indicating that it will reach USD 20.37 Billion by 2032. The market is expected to expand at a compound annual growth rate (CAGR) of 6.4% over the forecast period 2024-2032.
Asia Pacific will contribute lucratively towards the global market size over the estimated timeline. The regional market surge is due to massively evolved regulatory tools in countries such as Japan and Australia. Furthermore, supportive government policies for increasing FDAs and need for increasing approval of new products will steer regional market growth. In addition to this, necessity of relaxing laws related to procurement control will drive regional industry trends.
The key market participants include Integer Holdings, ICON Plc, Medpace, Emergo, IQVIA Holdings, Intertek Plc, Covance, Promedica International, Freyr, and SGS SA.
Choose License Type
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed