Medical Devices Vigilance Market Size, Share, Growth, Trends, and Forecast, 2028

Medical Devices Vigilance Market- by Delivery mode (On-demand, On-premise), by Application (Diagnostic, Surgical, Therapeutic, Research sectors), by End-user (Clinical research organizations, Original equipment manufacturers, Business process outsourcing) - Global Industry Perspective Comprehensive Analysis and Forecast, 2020 - 2026
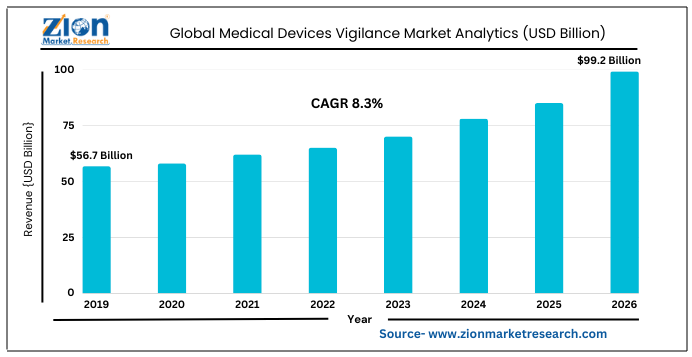
The global medical devices vigilance market was worth $56.7 billion in 2019, and is predicted to reach $99.2 billion by 2026, increasing at a CAGR of 8.3%.
According to the report, global demand for Medical Devices Vigilance market was valued at approximately USD 56.7 Billion in 2019, and is expected to generate revenue of around USD 99.2 Billion by end of 2026, growing at a CAGR of around 8.3 % between 2020 and 2026.
Global Medical Devices Vigilance Market: Overview
With the testing of medical equipment becoming necessary for the healthcare industry, medical device vigilance is gaining prominence across the globe. The recognition of potential hazards pertaining to medical instruments is included in medical device vigilance. The vigilance market for medical devices can be traced back to 1992, when the Global Harmonization Task Force (GHTF) was formed to standardize national regulatory frameworks for medical devices. The goal was to improve access to effective, secure and clinically useful medical technologies.
Global Medical Devices Vigilance Market: Growth Factors
The surge in the number of medical devices recalls due to safety issues is the key factor driving the global market growth during the forecast period. Additionally, growing awareness among people pertaining to availability of medical device vigilance software and reporting of adverse events will positively leverage medical devices vigilance market growth over the analysis timeline.
Furthermore, favorable government scenario pertaining to medical devices vigilance ensuing patient safety will serve as an impact rendering factor that will bolster the business scope. Increasing burden on medical devices manufacturers to produce safe medical equipment as well as strict safety laws put into practice/use by regulatory bodies pertaining to pre- and post-commercialization of medical equipment will further spur the market revenue. However, carelessness of production units towards the maintenance of product safety may refrain the growth of medical devices vigilance market over the upcoming years.
Global Medical Devices Vigilance Market: Segmentation
The global medical devices vigilance market can be classified into the delivery mode, application, and end-user. Based on the delivery mode, the market is sectored into on-demand and on-premise. On the basis of application, the market is segmented into diagnostic, surgical, therapeutic, and research sectors. Based on the end-user, the medical devices vigilance market is classified into clinical research organizations, original equipment manufacturers, and business process outsourcing.
Global Medical Devices Vigilance Market: Regional Analysis
Based on the region, the global medical devices vigilance market can be divided into five main regions: North America, Latin America, Europe, Asia Pacific, and the Middle East and Africa. North America is likely to dominate the global medical devices vigilance market growth over the years ahead, owing to the tremendous demand for medical devices vigilance in the region. A massive number of reported hostile activities will elevate the acceptance of vigilance systems. A strong base of medical equipment producing firms in North America will further augment regional business growth. Moreover, Asia Pacific medical devices vigilance market will register a profitable growth over the years to come, owing to the growing focus of medical device manufacturers on product safety. Additionally, the presence of a large and diverse patient pool in the region coupled with a surge in clinical research outsourcing will further accelerate regional market growth.
Global Medical Devices Vigilance Market: Report Scope:
| Report Attributes | Report Details |
|---|---|
| Report Name | Medical Devices Vigilance Market |
| Market Size in 2019 | USD 56.7 Billion |
| Market Forecast in 2026 | USD 99.2 Billion |
| Compound Annual Growth Rate | CAGR of 8.3% |
| Number of Pages | 110 |
| Forecast Units | Value (USD Billion), and Volume (Units) |
| Key Companies Covered | PZEINCRO, Sparta Systems, AssurX, Oracle, Xybion, INTEL, Sarjen Systems, MDI Consultants, AB-Cube, Numerix, and Omnify Software. |
| Segments Covered | By Product Type, By Application, And By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latian America, Middle East and Africa (MEA) |
| Countries Covered | North America: U.S and Canada Europe: Germany, Italy, Russia, U.K, Spain, France, Rest of Europe APAC: China, Australia, Japan, India, South Korea, South East Asia, Rest of Asia Pacific Latin America: Brazil, Argentina, Chile The Middle East And Africa: South Africa, GCC, Rest of MEA |
| Base Year | 2019 |
| Historical Year | 2016 to 2018 |
| Forecast Year | 2019 - 2026 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
To know more about this report, request a sample copy.
The medical devices vigilance market in Europe is slated to experience rapid growth, subject to stringent medical device regulations and policies in countries such as the UK. Latin America and the Middle East and Africa possess brighter market growth prospects and are likely to contribute considerably towards the global market share in the upcoming years.
Global Medical Devices Vigilance Market: Competitive Players
Some of the key players in medical devices vigilance market are ZEINCRO, Sparta Systems, AssurX, Oracle, Xybion, INTEL, Sarjen Systems, MDI Consultants, AB-Cube, Numerix, and Omnify Software.
The report segments the Global Medical Devices Vigilance market as follows:
Global Medical Devices Vigilance Market: Delivery mode Segment Analysis
-
On-demand
- On-premise
Global Medical Devices Vigilance Market: Application Segment Analysis
- Diagnostic
- Surgical
- Therapeutic
- Research sectors
Global Medical Devices Vigilance Market: End-user Segment Analysis
- Clinical research organizations
- Original equipment manufacturers
- Business process outsourcing
Global Medical Devices Vigilance Market: Regional Segment Analysis
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
The Global Medical Devices Vigilance Market was valued at USD USD 56.7 Billion in 2019.
The Global Medical Devices Vigilance Market is expected to reach USD USD 99.2 Billion by 2026, growing at a CAGR of 8.3% between 2018 to 2025.
The surge in the number of medical devices recalls due to safety issues is the key factor driving the global market growth during the forecast period. Additionally, growing awareness among people pertaining to availability of medical device vigilance software and reporting of adverse events will positively leverage medical devices vigilance market growth over the analysis timeline.
The medical devices vigilance market in Europe is slated to experience rapid growth, subject to stringent medical device regulations and policies in countries such as the UK. Latin America and the Middle East and Africa possess brighter market growth prospects and are likely to contribute considerably towards the global market share in the upcoming years.
ZEINCRO, Sparta Systems, AssurX, Oracle, Xybion, INTEL, Sarjen Systems, MDI Consultants, AB-Cube, Numerix, and Omnify Software.
Choose License Type
List of Contents
Global Medical Devices Vigilance OverviewGlobal Medical Devices Vigilance Growth FactorsGlobal Medical Devices Vigilance SegmentationGlobal Medical Devices Vigilance Regional AnalysisGlobal Medical Devices Vigilance Report Scope:Global Medical Devices Vigilance Competitive PlayersThe report segments the Global Medical Devices Vigilance market as follows:Global Medical Devices Vigilance Delivery mode Segment AnalysisGlobal Medical Devices Vigilance Application Segment AnalysisGlobal Medical Devices Vigilance End-user Segment AnalysisGlobal Medical Devices Vigilance Regional Segment AnalysisRelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed