Multi-Cancer Early Detection Market Size, Share, Analysis, Trends, Growth, 2032

Multi-Cancer Early Detection Market By End-Use (Diagnostic Laboratories and Hospitals), By Type (Gene Panel, LDT, & Others and Liquid Biopsy), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2024 - 2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 1,065.90 Million | USD 4,200.10 Million | 16.58% | 2023 |
Multi-Cancer Early Detection Industry Prospective:
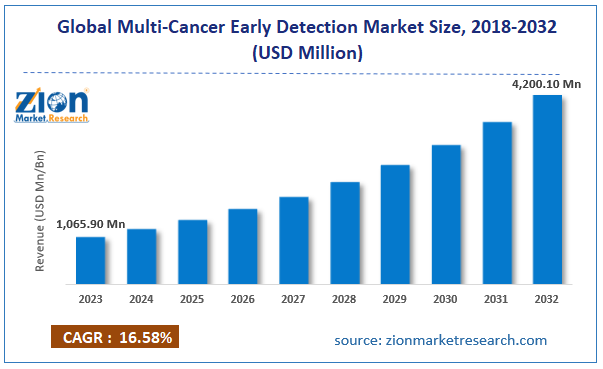
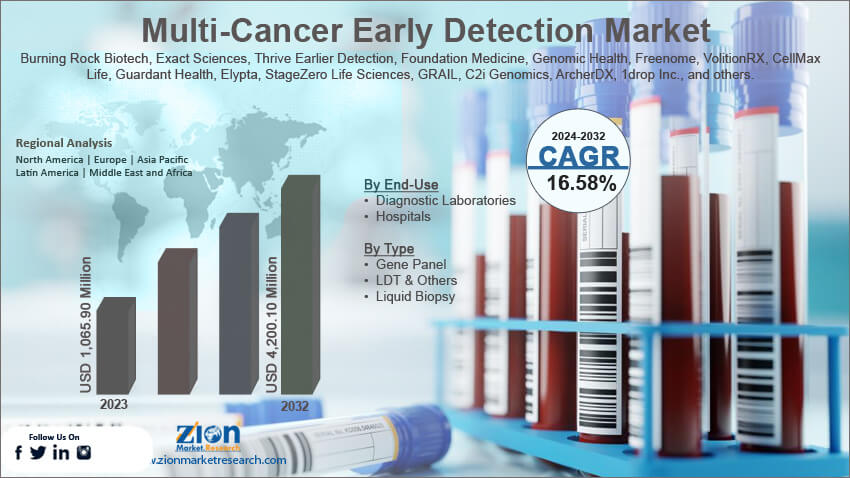
The global multi-cancer early detection market size was worth around USD 1,065.90 million in 2023 and is predicted to grow to around USD 4,200.10 million by 2032 with a compound annual growth rate (CAGR) of roughly 16.58% between 2024 and 2032.
Multi-Cancer Early Detection Market: Overview
Multi-cancer early detection (MCED) is an innovative and revolutionary cancer screening test leveraging the potential of machine learning and genome science for ear; the detection of multiple types of cancer using a single blood sample. MCED tests are a type of liquid biopsy that intends to catch cancer at its early stages when prevention of treatment is possible or before the symptoms start to appear. These tests are powered by advanced machine learning algorithms as they will be used for identifying tumor origin based on protein and deoxyribonucleic acid profiles. The blood sample drawn from the patients will be used for testing specific DNA pieces or proteins that are associated with cancer cells. In some cases, if the test hints at the presence of these profiles, it means that the patient has a certain type of cancer. Additionally, the test will also be able to help medical professionals understand the origin organ of the cancer cells. The form of cancer screening will allow the healthcare sector to use measures to detect cancer before it spreads to the body and becomes difficult to treat. Early cancer detection is one of the crucial ways in which cancer can be efficiently treated. Early detection allows patients a greater chance of beating otherwise intractable conditions. An additional benefit of MCED tests is their ability to detect the presence of multiple types of cancer using a single sample, unlike traditional ways of cancer screening. The forecast period has high growth potential for the multi-cancer early detection industry.
Key Insights:
- As per the analysis shared by our research analyst, the global multi-cancer early detection market is estimated to grow annually at a CAGR of around 16.58% over the forecast period (2024-2032)
- In terms of revenue, the global multi-cancer early detection market size was valued at around USD 1,065.90 million in 2023 and is projected to reach USD 4,200.10 million, by 2032.
- The market is projected to grow at a significant rate due to the growing demand for effective cancer screening tools
- Based on the end-use, the hospital segment is growing at a high rate and will continue to dominate the global market as per industry projection
- Based on the type, the gene panel, LDT, & others segment is anticipated to command the largest market share
- Based on region, North America is projected to dominate the global market during the forecast period
 Request Free Sample
Request Free Sample
Multi-Cancer Early Detection Market: Growth Drivers
Growing demand for effective cancer screening tools will drive the market growth rate
The global multi-cancer early detection market is expected to witness high growth due to the increasing demand for effective cancer screening tools. According to leading regional and international healthcare agencies, cancer is one of the leading causes of medically-caused deaths across the globe. The World Health Organization (WHO) reported over 10 million cancer-related deaths in 2020. Apart from acting as a cause of death, the condition is difficult to detect in early stages and treat when it reaches higher levels. The primary reason causing difficulty in cancer detection is the ability of the cancer cells to hide from the immune system. With time, they continue to grow rapidly and become out of control after some time. Studies indicate that while certain types of cancer are relatively easier to develop, the healthcare sector lacks access to early cancer screening or detection tests for most types.
For instance, in the majority of cases, women are unable to detect ovarian cancer until it reaches stage 3 or stage 4 thus making it the deadliest gynecological cancer. It drastically reduces the chance of survival among women. Another cancer type difficult to detect is the tumors of the pancreas due to the location of the organ within the body. Such drastic statistics have resulted in the need to develop more efficient cancer screening and detection tests that can alert the patient about the condition at early stages. According to Cancer Research UK, 6 out of 10 patients with lung cancer can survive the disease for 5 or more years if it is caught at its earliest stage.
Increasing awareness among the general population about cancer and its detection measures will deliver higher product adoption
The general population is increasingly becoming aware of cancer prevalence. In addition to this, people are actively seeking solutions that can help them prevent the disease or detect it as early as possible. This can be achieved by regularly undergoing checkups and screening tests. However, it is nearly impossible to undergo cancer screening tests frequently for all types of cancer. In such cases, the global multi-cancer early-detection market offerings will prove beneficial. In August 2023, leading pharmaceutical giant AstraZeneca launched Cancer Care Africa to help the continent beat the burgeoning pressure of cancer patients.
Multi-Cancer Early Detection Market: Restraints
Lack of approval from regulatory bodies could limit the market adoption rate
The global industry for multi-cancer early detection could be restricted due to the lack of approval from regulatory bodies for mass applications. For instance, the United States Food and Drugs Administration has not approved or cleared several MCED tests and they fall under the Clinical Laboratory Improvement Act (CLIA) in the country. Which means that they can only be used if the medical professional deems it fit for use. Similarly, other countries are lacking approval rates thus effectively slowing down the market adoption rate.
Multi-Cancer Early Detection Market: Opportunities
Higher investments in cancer research could generate extensive growth opportunities during the forecast period
The global multi-cancer early detection market is expected to gain from the increasing investments in cancer research and the development of new diagnostic measures and treatment processes. Cancer research funding has improved significantly in the last decade as regional governments, international healthcare agencies, and private companies are actively participating in activities that could deliver positive results. In March 2024, the Union for International Cancer Control (UICC) announced that it was evaluating applications for implementing grants on cancer research to prevent the impact of the disease in Europe. The UICC initiative was launched in 2022 at the World Cancer Congress. The agency will provide 500,000 Euros in grants with a duration of two years. In February 2024, leading pharmaceutical company Pfizer Inc. and The American Cancer Society announced the launch of Change the Odds: Uniting to Improve Cancer Outcomes™. It is a three-year-long initiative and will aim toward bridging the gap in disparities that exist between cancer care. Pfizer has invested $15 million in improving health outcomes in unprivileged societies in the US.
Rising efforts toward mainstreaming MCED tests will deliver significant results
Multi-cancer early detection tests have the potential to change the course of cancer detection and treatment. This has resulted in increased efforts toward making the novel cancer screening method more mainstream and improving patient accessibility. In February 2024, the National Institutes of Health (NIH) launched a clinical trials network for evaluating flourishing technologies for cancer screening. The Cancer Screening Research Network (CSRN) and the Cancer Moonshot? will work in tandem to explore ways to identify cancer at early stages thus opening new doors for growth in the global multi-cancer early detection market.
Multi-Cancer Early Detection Market: Challenges
Challenges in the widespread roll-out of MCED tests could hamper the market expansion rate
The global industry for multi-cancer early detection is expected to face challenges when industry players try to achieve a smooth wide-spread MCED test roll-out. The initial challenge will be in terms of associated high expenses. Moreover, these tests are currently not covered by medical insurance which may further limit the use of the tests among financially unstable segments of the society.
Multi-Cancer Early Detection Market: Segmentation
The global multi-cancer early detection market is segmented based on end-use, type, and region.
Based on the end-use, the global market segments are diagnostic laboratories and hospitals. In 2023, the highest demand was observed in the hospitals segment. Currently, these advanced technologies are used in hospitals as they treat several types of cancer under one roof. In 2023, the segment dominated nearly 48.5% of the total share. Furthermore, at present, MCED tests are only conducted after approval from the medical practitioners. The rising number of cancer-care hospitals globally as well as increased funding in these facilities to deploy state-of-the-art architecture will help the segment thrive.
Based on the type, the global market segments are gene panel, LDT, & others, and liquid biopsy. In 2023, the segment with the highest revenue share was gene panel, LDT, & others. It dominated nearly 98.2% of the overall revenue. This is because laboratory-developed tests (LDT) can be used across hospitals and treatment facilities without receiving approval from the FDA. During the forecast period, the liquid biopsy segment is projected to generate significant results as more players enter the market and invest in the development of new tests.
Multi-Cancer Early Detection Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Multi-Cancer Early Detection Market |
| Market Size in 2023 | USD 1,065.90 Million |
| Market Forecast in 2032 | USD 4,200.10 Million |
| Growth Rate | CAGR of 16.58% |
| Number of Pages | 228 |
| Key Companies Covered | Burning Rock Biotech, Exact Sciences, Thrive Earlier Detection, Foundation Medicine, Genomic Health, Freenome, VolitionRX, CellMax Life, Guardant Health, Elypta, StageZero Life Sciences, GRAIL, C2i Genomics, ArcherDX, 1drop Inc., and others. |
| Segments Covered | By End-Use, By Type, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Multi-Cancer Early Detection Market: Regional Analysis
Market growth will be the highest in North America during the forecast period
The global multi-cancer early detection market will be led by North America during the projection period. In 2023, the region dominated 35% of the global market share. The growing number of cancer patients in the US region is driving the demand for effective cancer diagnostic and treatment solutions. As per the American Cancer Society, more than 1.9 million people in the US were diagnosed with cancer in 202. Moreover, the US and Canada regions have higher patient awareness about cancer and the importance of early detection, the robust healthcare infrastructure of these countries further helps the region flourish.
The US, for instance, hosts several cancer patients from across the globe seeking cancer-related treatment. Furthermore, the region is registering immense research investments to develop infrastructure that supports extensive research on multi-cancer early detection. In February 2024, UC San Francisco announced the launch of the Cancer Early Detection and Interception (CEDI) initiative that aims to detect cancer in its early stages and prevent its progress in the body. In September 2023, Eone-Diagnomics Genome Center (EDGC), based in the US, was one step away from developing a blood-based multi-cancer early detection test for detecting dozens of cancer types and also has non-cancer applications such as the progression of Alzheimer's disease.
Multi-Cancer Early Detection Market: Competitive Analysis
The global multi-cancer early detection market is led by players like:
- Burning Rock Biotech
- Exact Sciences
- Thrive Earlier Detection
- Foundation Medicine
- Genomic Health
- Freenome
- VolitionRX
- CellMax Life
- Guardant Health
- Elypta
- StageZero Life Sciences
- GRAIL
- C2i Genomics
- ArcherDX
- 1drop Inc.
The global multi-cancer early detection market is segmented as follows:
By End-Use
- Diagnostic Laboratories
- Hospitals
By Type
- Gene Panel
- LDT & Others
- Liquid Biopsy
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Multi-cancer early detection (MCED) is an innovative and revolutionary cancer screening test leveraging the potential of machine learning and genome science for ear; the detection of multiple types of cancer using a single blood sample.
The global multi-cancer early detection market is expected to witness high growth due to the increasing demand for effective cancer screening tools.
According to study, the global multi-cancer early detection market size was worth around USD 1,065.90 million in 2023 and is predicted to grow to around USD 4,200.10 million by 2032.
The CAGR value of multi-cancer early detection market is expected to be around 16.58% during 2024-2032.
The global multi-cancer early detection market will be led by North America during the projection period.
The global multi-cancer early detection market is led by players like Burning Rock Biotech, Exact Sciences, Thrive Earlier Detection, Foundation Medicine, Genomic Health, Freenome, VolitionRX, CellMax Life, Guardant Health, Elypta, StageZero Life Sciences, GRAIL, C2i Genomics, ArcherDX and 1drop Inc.
The report explores crucial aspects of the multi-cancer early detection market including detailed discussion of existing growth factors and restraints while also browsing future growth opportunities and challenges that impact the market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed