Pediatric Clinical Trials Market Size, Share, Trends, Growth and Forecast 2034

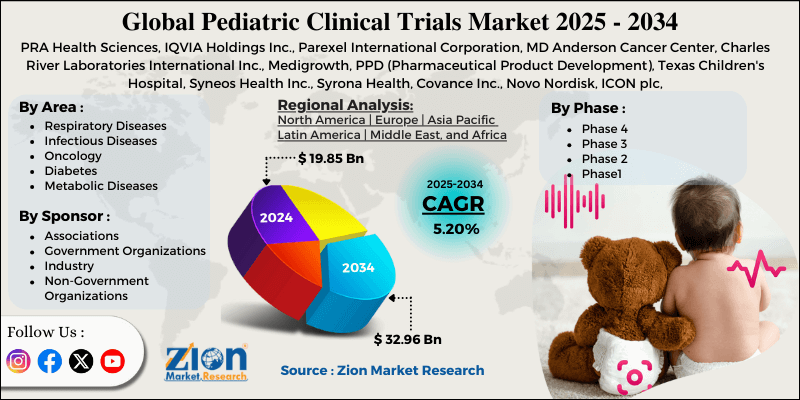
Pediatric Clinical Trials Market By Area (Respiratory Diseases, Infectious Diseases, Oncology, Diabetes, Metabolic Diseases, HIV, Cardiovascular Diseases, and Others), By Sponsor (Associations, Government Organizations, Industry, Non-Government Organizations, and Others), By Phase (Phase 4, Phase 3, Phase 2, and Phase1), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 19.85 Billion | USD 32.96 Billion | 5.20% | 2024 |
Pediatric Clinical Trials Industry Prospective:
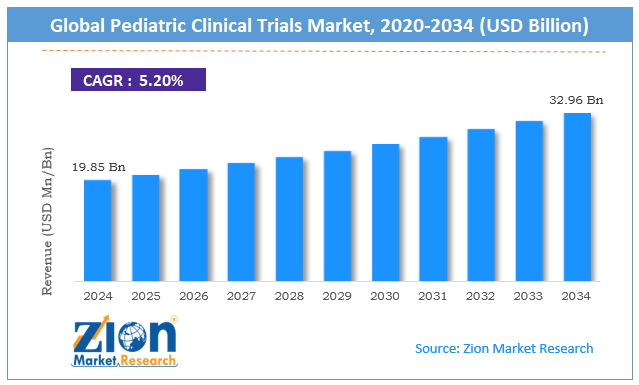
The global pediatric clinical trials market size was worth around USD 19.85 billion in 2024 and is predicted to grow to around USD 32.96 billion by 2034, with a compound annual growth rate (CAGR) of roughly 5.20% between 2025 and 2034.
Pediatric Clinical Trials Market: Overview
Pediatric clinical trials are drug discovery and development-oriented procedures that aim to develop child-specific medicines and treatment plans. Children and their medical needs are significantly different from those of adults since they have distinct physiological and developmental differences.
Pediatric clinical trials are necessary for developing age-specific therapies and medical interventions to ensure that the best medical care is available to children. Pediatric clinical trials are specially designed to ensure that children are safe during the trial process.
For instance, the World Health Organization (WHO) has developed the International Clinical Trials Registry Platform (ICTRP), which focuses on promoting the conduct of relevant and ethical clinical trials. Special clinical considerations are taken into account during pediatric clinical trials.
During the forecast period, the global industry for pediatric clinical trials is expected to grow rapidly, driven by increasing demand for improved medical care for children of all ages.
In addition, growing cases of rare and fatal diseases being diagnosed among children may further help the industry gain growth momentum. A key factor that could limit the industry revenue is growing ethical and safety concerns related to children being involved in clinical trials.
Key Insights:
- As per the analysis shared by our research analyst, the global pediatric clinical trials market is estimated to grow annually at a CAGR of around 5.20% over the forecast period (2025-2034)
- In terms of revenue, the global pediatric clinical trials market size was valued at around USD 19.85 billion in 2024 and is projected to reach USD 32.96 billion by 2034.
- The pediatric clinical trials market is projected to grow at a significant rate due to the growing cases of pediatric disorders and medical conditions.
- Based on the area, the oncology diseases segment is growing at a high rate and will continue to dominate the global market as per industry projections.
- Based on the Phase, the Phase 3 segment is anticipated to command the largest market share.
- Based on region, North America is projected to dominate the global market during the forecast period.
Pediatric Clinical Trials Market: Growth Drivers
Growing cases of pediatric disorders and medical conditions act as an essential market growth driver
The global pediatric clinical trials market is expected to be driven by the rising cases of pediatric disorders and medical conditions reported worldwide.
Some common medical problems in children include skin-related conditions, flu, colds, ear infections, bronchitis, and diarrhea. In addition to this, there is a growing rate of more serious conditions being diagnosed among children of all age groups. For instance, recent statistics indicate an alarming increase in cancer among children.
According to the WHO, more than 400,000 children across the globe between the ages of 0 and 19 years are diagnosed with cancer. The fatality rate among pediatric patients due to brain tumors, leukemia, and lymphomas is growing every day.
Further rare genetic conditions, such as Rett syndrome, Maple Syrup Urine Disease (MSUD), Krabbe disease, and a thousand other diseases, have shown an increase in diagnosis. The demand for children-specific medical treatments has been on the rise in the last few years, encouraging pediatric clinical trials rate simultaneously.
Improving awareness rate about the benefits of clinical trials for children to open new avenues for growth
Pharmaceutical companies and government organizations, along with other relevant regulatory bodies, have amplified projects and initiatives concerning awareness related to the importance of holding pediatric clinical trials. The medical needs of children are significantly different from those of adults.
Medical interventions suited for the healthcare needs of children must be developed for effective results. For instance, in February 2024, Innovative Trials, a leading specialist in clinical trial patient recruitment and retention acceleration, announced the launch of a new initiative to promote clinical trial patient retention among children.
The company has collaborated with the US charity Oliver Patch Project for the launch of the Retention Patch Program. The global pediatric clinical trials market is expected to benefit from such initiatives in the long term since they raise awareness and clarify any misconceptions that may exist in potential pediatric studies or their guardians.
Pediatric Clinical Trials Market: Restraints
Concerns over children's safety and the high cost of investment to limit market growth rate in the future
The global pediatric clinical trials industry is expected to be restricted due to the growing concerns over the physical and mental well-being of children undergoing trials.
Since the drugs for pediatric medical care are still in the research & development phase, there are chances that the medical intervention may lead to serious side effects, including death in rare cases. Furthermore, the high cost of investment required for setting up compliant pediatric clinical trials may further dilute market revenue in the long run.
Pediatric Clinical Trials Market: Opportunities
Ongoing advancements in the clinical trials for children to generate growth opportunities in the future
The global pediatric clinical trials market is expected to be led by the ongoing advancements and use of modern technologies for conducting clinical trials in children.
For instance, in December 2024, Sidra Medicine, a leading member of the Qatar Foundation, announced the launch of the country’s first industry-sponsored pharmaceutical trial for pediatric congenital hyperinsulinism on a young patient. Sidra Medicine has recently launched a Clinical Trials Program, as the brand aims to provide enhanced medical care for children with complex and rare diseases.
Pfizer, one of the world’s leading pharmaceutical companies, launched the Pediatric Center of Excellence in 2014. The company aims to better understand the needs of children participating in clinical trials to improve the final outcomes of the trial projects.
In December 2024, Novartis announced the positive results obtained from the Phase III STEER study, which assessed the effectiveness of intrathecal onasemnogene abeparvovec (OAV101 IT) in treating children aged two to less than 18 years with spinal muscular atrophy (SMA) Type 2.
Furthermore, market players are investing in integrating advanced technologies such as virtual reality (VR) and augmented reality (AR) in pediatric medical trials to improve testing outcomes.
Pediatric Clinical Trials Market: Challenges
Ethical concerns over the consent of patients and their guardians to challenge the market expansion
The global pediatric clinical trials industry is expected to be challenged by ethical concerns over obtaining consent from young patients and their guardians.
For instance, children and their parents or guardians may not fully understand the risks associated with participating in clinical trials. Furthermore, the regulatory complexities of pediatric clinical trials changing from one region to another may complicate the market growth trajectory, according to studies.
Pediatric Clinical Trials Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Pediatric Clinical Trials Market |
| Market Size in 2024 | USD 19.85 Billion |
| Market Forecast in 2034 | USD 32.96 Billion |
| Growth Rate | CAGR of 5.20% |
| Number of Pages | 215 |
| Key Companies Covered | PRA Health Sciences, IQVIA Holdings Inc., Parexel International Corporation, MD Anderson Cancer Center, Charles River Laboratories International Inc., Medigrowth, PPD (Pharmaceutical Product Development), Texas Children's Hospital, Syneos Health Inc., Syrona Health, Covance Inc., Novo Nordisk, ICON plc, KCR, Medpace Holdings Inc., and others. |
| Segments Covered | By Area, By Sponsor, By Phase, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Pediatric Clinical Trials Market: Segmentation
The global pediatric clinical trials market is segmented based on area, sponsor, phase, and region.
Based on the area, the global market segments are respiratory diseases, infectious diseases, oncology diseases, metabolic diseases, HIV, cardiovascular diseases, and others.
In 2024, the highest growth was listed in the oncology segment, which dominated nearly 21.81% of the total revenue. The segmental growth is expected to be driven by emerging opportunities for strategic partnerships between stakeholders and increased demand for effective oncology-related medical interventions.
Based on sponsor, the global pediatric clinical trials industry is divided into associations, government organizations, industry, non-government organizations, and others.
Based on the phase, the global market segments are Phase 4, Phase 3, Phase 2, and Phase 1. In 2024, the highest growth was listed in the Phase 3 segment, which held over 47% of the final revenue. The presence of strict regulatory guidelines in Phase 3 promotes the faster launch of novel drug therapies and discoveries. In 2024, the year-on-year growth in the Phase 3 segment was over 8.25%.
Pediatric Clinical Trials Market: Regional Analysis
North America is to hold prominence over other markets during the forecast period
The global pediatric clinical trials market will be led by North America during the forecast period. The US will emerge as the highest revenue-generating region and may hold command over 15.05% of the regional market share by the end of the projection period. The presence of a robust and world-class healthcare and pharmaceutical infrastructure in the region will facilitate the US dominance over other countries.
Moreover, the US pediatric clinical trials sector is well-organized and guided by comprehensive laws, which ease the process of compliance among regional players. The role of patient advocacy organizations in the region will be to shape the region’s overall growth trajectory.
Europe is another flourishing market with high growth potential. Countries such as France, Germany, and the UK will help Europe obtain a revenue share of over 14% in the global market. The growing number of pediatric patients across European countries and an increasing number of pediatric trials in the region will encourage higher revenue.
In March 2025, the Clinical Trials Information System launched a new clinical trial map for its public website, providing real-time information on clinical trials conducted in the area and increasing access to clinical research throughout the European Union.
Pediatric Clinical Trials Market: Competitive Analysis
The global pediatric clinical trials market is led by players like:
- PRA Health Sciences
- IQVIA Holdings Inc.
- Parexel International Corporation
- MD Anderson Cancer Center
- Charles River Laboratories International Inc.
- Medigrowth
- PPD (Pharmaceutical Product Development)
- Texas Children's Hospital
- Syneos Health Inc.
- Syrona Health
- Covance Inc.
- Novo Nordisk
- ICON plc
- KCR
- Medpace Holdings Inc.
The global pediatric clinical trials market is segmented as follows:
By Area
- Respiratory Diseases
- Infectious Diseases
- Oncology
- Diabetes
- Metabolic Diseases
- HIV
- Cardiovascular Diseases
- Others
By Sponsor
- Associations
- Government Organizations
- Industry
- Non-Government Organizations
- Others
By Phase
- Phase 4
- Phase 3
- Phase 2
- Phase1
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Pediatric clinical trials are drug discovery and development-oriented procedures that aim to develop child-specific medicines and treatment plans.
The global pediatric clinical trials market is expected to be driven by the rising cases of pediatric disorders and medical conditions reported worldwide.
According to study, the global pediatric clinical trials market size was worth around USD 19.85 billion in 2024 and is predicted to grow to around USD 32.96 billion by 2034.
The CAGR value of the pediatric clinical trials market is expected to be around 5.20% during 2025-2034.
The global pediatric clinical trials market will be led by North America during the forecast period.
The global pediatric clinical trials market is led by players like PRA Health Sciences, IQVIA Holdings, Inc., Parexel International Corporation, MD Anderson Cancer Center, Charles River Laboratories International Inc., Medigrowth, PPD (Pharmaceutical Product Development), Texas Children's Hospital, Syneos Health Inc., Syrona Health, Covance Inc., Novo Nordisk, ICON plc, KCR, and Medpace Holdings, Inc.
The report explores crucial aspects of the pediatric clinical trials market, including a detailed discussion of existing growth factors and restraints, while browsing future growth opportunities and challenges that impact the market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed