Pharmacovigilance Market Size, Share, Trends, Growth 2032

Pharmacovigilance Market By Clinical Trial Phase (Pre-Clinical, Phase 1, Phase 2, Phase 3, and Phase 4), By Service Provider (In-House and Contract Outsourcing), By Method (Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event Monitoring, and EHR Mining), and By End-User (Hospitals, Research Organizations, and Industries), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2024 - 2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 9.20 Billion | USD 18.83 Billion | 8.28% | 2023 |
Pharmacovigilance Industry Prospective:
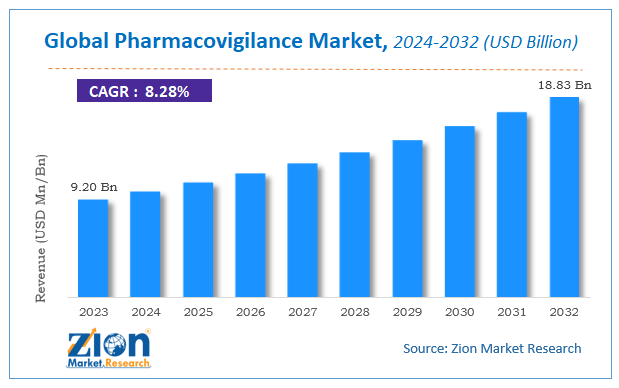
The global pharmacovigilance market size was worth around USD 9.20 billion in 2023 and is predicted to grow to around USD 18.83 billion by 2032 with a compound annual growth rate (CAGR) of roughly 8.28% between 2024 and 2032.
In order to give the users of this report a comprehensive view of the pharmacovigilance market, we have included a competitive landscape and an analysis of Porter’s Five Forces model for the market. The study encompasses a market attractiveness analysis, wherein all the segments are benchmarked based on their market size, growth rate, and general attractiveness.
Pharmacovigilance Market: Growth Drivers
The study of efficacy and safety in drugs and medical products is known as pharmacovigilance. Pharmacovigilance is the science that deals with collecting, detecting, assessing, and preventing adverse effects of medical products or drugs. It also analyzes the drugs’ side-effects and determines the ones that negatively affect the drug’s efficacy. It comprises of preclinical research, drug discovery and development, clinical research, post-marketing surveillance, etc. Every phase of the drug cycle is traced in pharmacovigilance, starting from its preclinical development stage to post-marketing surveillance.
The global pharmacovigilance market is driven by the rising awareness about the safety and efficacy of drugs among the worldwide population and the growing demand for substantially adopting safe medical practices. Emerging economies, such as India, China, and Brazil, have gathered momentum due to the increasing government support to open pharmacovigilance centers. This is further driving this global market’s growth. Additionally, the implementation of stringent post-market monitoring mechanism set by various government agencies for effective drug regulation system is also catalyzing the global pharmacovigilance market. The rising prevalence of adverse reactions caused due by drugs has inclined the biotechnological and pharmaceutical industries to adapt pharmacovigilance practices, which, in turn, is also expected to fuel this market in the future.
The report provides company market share analysis to give a broader overview of the key players in the market. In addition, the report also covers key strategic developments of the market including acquisitions & mergers, new product launch, agreements, partnerships, collaborations & joint ventures, research & development, product, and regional expansion of major participants involved in the market.
Pharmacovigilance Market: Segmentation
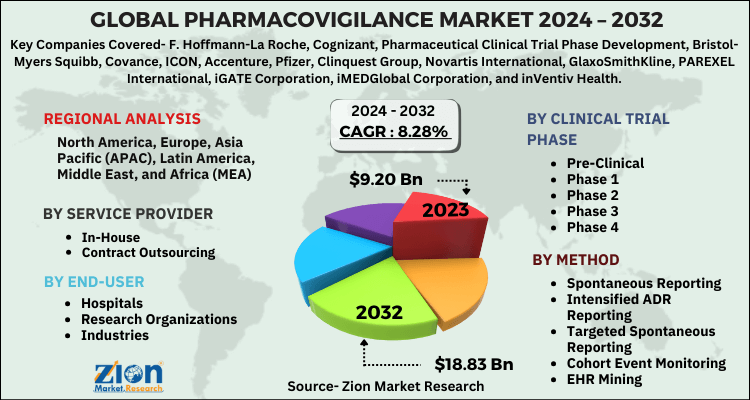
The study provides a decisive view on the pharmacovigilance market by segmenting it on the basis of clinical trial phase, service provider, method, end-user, and region.
By clinical trial phase, the market includes pre-clinical, phase 1, phase 2, phase 3, and phase 4.
By service provider, the pharmacovigilance market includes in-house and contract outsourcing.
By method, the market involves intensified ADR reporting, targeted spontaneous reporting, spontaneous reporting, cohort event monitoring, and EHR mining.
The end-user segment of the pharmacovigilance market is divided into hospitals, research organizations, and industries.
Pharmacovigilance Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Pharmacovigilance Market |
| Market Size in 2023 | USD 9.20 Billion |
| Market Forecast in 2032 | USD 18.83 Billion |
| Growth Rate | CAGR of 8.28% |
| Number of Pages | 110 |
| Key Companies Covered | F. Hoffmann-La Roche, Cognizant, Pharmaceutical Clinical Trial Phase Development, Bristol-Myers Squibb, Covance, ICON, Accenture, Pfizer, Clinquest Group, Novartis International, GlaxoSmithKline, PAREXEL International, iGATE Corporation, iMEDGlobal Corporation, and inVentiv Health |
| Segments Covered | By clinical trial phase, By service provider, By method, By end-user and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Pharmacovigilance Market: Regional Analysis
The regional segment includes the current and forecast demand for North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa with its further classification into major countries including the U.S., UK, Germany, France, China, Japan, India, Brazil, etc.
Europe is anticipated to hold a major share of the global pharmacovigilance market in the years ahead, due to the introduction of new laws by the European Union in 2012, which ensures good vigilance practices for medicine regulators and pharmaceutical companies. Rising prevalence of adverse drug reactions and the introduction of the new legislation have augmented the adoption of pharmacovigilance in many companies, which is fueling the European pharmacovigilance market growth. North America is estimated to hold the second largest share of the pharmacovigilance market globally in the future, due to the increasing number of research and development activities, substantial developments of new drugs, and flourishing biotechnology and pharmaceutical sectors in the U.S.
Pharmacovigilance Market: Competitive Analysis
The global pharmacovigilance market is dominated by players like:
- F. Hoffmann-La Roche
- Cognizant
- Pharmaceutical Clinical Trial Phase Development
- Bristol-Myers Squibb
- Covance
- ICON
- Accenture
- Pfizer
- Clinquest Group
- Novartis International
- GlaxoSmithKline
- PAREXEL International
- iGATE Corporation
- iMEDGlobal Corporation
- inVentiv Health
This report segments the global pharmacovigilance market as follows:
Global Pharmacovigilance Market: Clinical Trial Phase Analysis
- Pre-Clinical
- Phase 1
- Phase 2
- Phase 3
- Phase 4
Global Pharmacovigilance Market: Service Provider Analysis
- In-House
- Contract Outsourcing
Global Pharmacovigilance Market: Method Analysis
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
Global Pharmacovigilance Market: End-User Analysis
- Hospitals
- Research Organizations
- Industries
Global Pharmacovigilance Market: Regional Analysis
- North America
- The U.S.
- Europe
- UK
- France
- Germany
- Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- Middle East and Africa
Table Of Content
Methodology
FrequentlyAsked Questions
The science and activities of pharmacovigilance are dedicated to the detection, assessment, comprehension, and prevention of adverse effects or any other drug-related issues. The primary objective of pharmacovigilance is to guarantee the safe and effective use of medications and enhance patient safety.
The demand for robust pharmacovigilance systems to monitor and ensure the safety of new medications and treatments is driven by the growth in pharmaceutical research and development, as well as an increase in clinical trials. The incidence of adverse drug reactions (ADRs) increases in tandem with the number of pharmaceuticals available on the market. This requires the implementation of comprehensive pharmacovigilance practices to mitigate and manage the risks associated with drug therapies.
The global pharmacovigilance market size was worth around USD 9.20 billion in 2023 and is predicted to grow to around USD 18.83 billion by 2032.
The global pharmacovigilance market a compound annual growth rate (CAGR) of roughly 8.28% between 2024 and 2032.
The regional segment includes the current and forecast demand for North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa with its further classification into major countries including the U.S., UK, Germany, France, China, Japan, India, Brazil, etc.
Some key players in the global pharmacovigilance market include as F. Hoffmann-La Roche, Cognizant, Pharmaceutical Clinical Trial Phase Development, Bristol-Myers Squibb, Covance, ICON, Accenture, Pfizer, Clinquest Group, Novartis International, GlaxoSmithKline, PAREXEL International, iGATE Corporation, iMEDGlobal Corporation, and inVentiv Health.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed