Retrovirus Testing Market Size, Share, Trends, Growth and Forecast 2028

Retrovirus Testing Market By Test Type (Infectivity Assay, Product-Enhanced Reverse Transcriptase (PERT) Assay, Co-Cultivation Assay, Transmission Electron Microscopy, Radio-Immune Assay, Western Blot Analysis, Immunofluorescence, Serological Tests), By Technique Type (High-Throughput Screening, Enzyme-Linked Immuno-Sorbent Assay (ELISA), Polymerase Chain Reaction (PCR)), By Sample Type (Blood, Serum, Body Fluids, Cells), By End-User (Hospitals, Clinics, Diagnostic Laboratories & Centers), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2022 - 2028-
| Market Size in 2021 | Market Forecast in 2028 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 182.4 Million | USD 456.1 Million | 16.5% | 2021 |
Retrovirus Testing Industry Prospective:
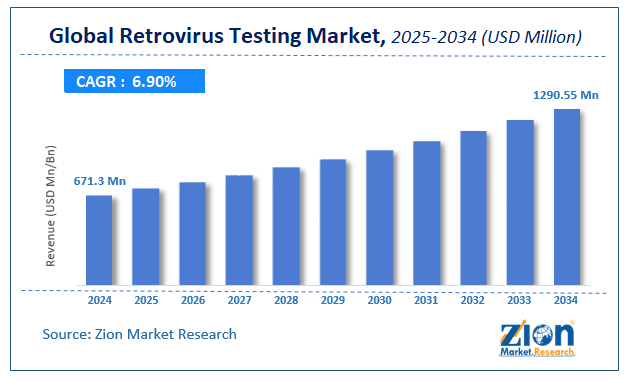
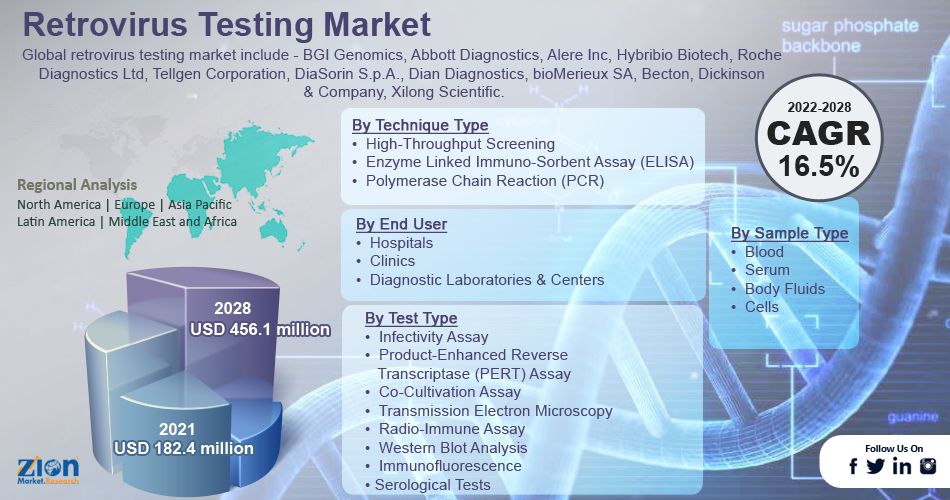
The global retrovirus testing market size was worth USD 182.4 million in 2021 and is estimated to grow to USD 456.1 million by 2028, with a compound annual growth rate (CAGR) of approximately 16.5 percent during the forecast period.
The report analyzes the retrovirus testing market's drivers, restraints/challenges, and their effect on the demands during the projection period. In addition, the report explores emerging opportunities in the retrovirus testing market.
Retrovirus Testing Market: Overview
A retrovirus is a single-stranded RNA virus that contains the reverse transcriptase enzyme. Once a retrovirus enters a host cell, reverse transcriptase helps the retroviral RNA be converted into retroviral DNA. The retrovirus then permits the chromosomal DNA of the host cell to incorporate the retroviral DNA. This integration facilitates retrovirus replication into the host genome, resulting in viral expression. HIV (Human Immunodeficiency Virus) is the most common retrovirus in humans. Other retroviruses that cause human infection involve Human T-cell Lymphotropic Virus-1 (HTLV-1), Human T-cell Lymphotropic Virus-2 (HTLV-2), HIV-1, HIV-2, and others. The rising incidence of HIV and increased awareness about HIV diagnosis drive the global retrovirus testing market. Retrovirus testing tools are one of the most effective tools for disease identification. These tools provide rapid results with high efficacy, enhancing the retrovirus testing industry.
The introduction of quick and technologically sophisticated methods for detecting retroviral infections, rising awareness of diagnostic tests, and rising expenditure associated with retrovirus screening are anticipated to drive the global market during the forecast period. The shortage of trained professionals to conduct the assays and the huge costs of retrovirus laboratory testing assay methods and diagnostic kits are expected to impede the market over the forecast period.
COVID-19 Impact:
The COVID-19 outbreak reduced the market share of retrovirus testing. As a result, the demand for HIV diagnostic procedures has significantly decreased, as have patient visits to healthcare facilities, which has negatively impacted the market growth of HIV diagnostics. As the total of COVID-19 patients decreased, demand for HIV diagnostics increased significantly in 2021. Sales of HIV medical testing in low-risk settings also expanded due to medical facilities implementing standard operating procedures and a reduced death rate associated with COVID-19.
Key Insights
- As per the analysis shared by our research analyst, the global retrovirus testing market value will grow at a CAGR of 16.5% over the forecast period.
- In terms of revenue, the global retrovirus testing market size was valued at around USD 182.4 million in 2021 and is projected to reach USD 456.1 million by 2028.
- The major factors driving the market's growth are the introduction of rapid and technologically sophisticated methods for detecting retroviral infections, rising awareness of diagnostic tests, and rising expenditure associated with retrovirus screening.
- By sample type, the blood category dominated the market in 2021.
- By end user, the diagnostic laboratories & centers segment dominated the market in 2021.
- By technique type, the high-throughput techniques and PCR-based amplification category dominated the market in 2021.
- North America dominated the global retrovirus testing market in 2021.
Retrovirus Testing Market: Growth Drivers
Rising government initiatives to increase the number of retrovirus tests to drive market growth
According to WHO, approximately 38 million people worldwide were infected with HIV in 2019. 68% of adults and 53% of children who have contracted the virus are receiving lifetime antiretroviral therapy (ART) as a result of various initiatives by the government and international organizations. Reimbursement policies and supportive government initiatives are additional major factors influencing global retrovirus testing market revenue growth. For instance, the Egyptian government announced in 2020 that it would provide confidential and free HIV diagnostics tests at examination and counseling centers in all 27 governorates. The introduction of rapid and technologically sophisticated methods for detecting retroviral infections, rising awareness of diagnostic tests, and rising costs associated with retrovirus screening are anticipated to drive the global market for retrovirus testing during the forecast period.
Retrovirus Testing Market: Restraints
The lack of skilled professionals to perform the assays and the high cost of retrovirus testing kits hinder the market growth
The lack of trained professionals to perform sensitive assays related to retrovirus testing and the high cost of assay methods & diagnostic kits are factors limiting the growth of the global retrovirus testing market. HIV stigma and the absence of adequate HIV reagents & kits may also limit the growth of the global market.
Retrovirus Testing Market: Segmentation
The global retrovirus testing market has been segmented into test type, technique type, sample type, and end-user.
Based on test type, the market is divided into infectivity assay, product-enhanced reverse transcriptase (PERT) assay, co-cultivation assay, transmission electron microscopy, radio-immune assay, western blot analysis, immunofluorescence, and serological tests. Among these, infectivity assays are the most frequently used method for retrovirus testing in the global market due to the rapid and accurate results obtained from these assays.
Based on technique type, the market is classified into high-throughput screening, enzyme-linked immunosorbent assay (ELISA), and polymerase chain reaction (PCR). In 2021, the high-throughput techniques and PCR category dominated the global market. High-throughput techniques and PCR-based amplification are primarily used in retrovirus testing to detect retrovirus infectious diseases in the target genome with high sensitivity.
Based on sample type, the market is classified into blood, serum, body fluids, and cells. The blood segment dominated the market in 2021, owing to the increased blood transfusions and donations.
Based on end-user, the global retrovirus testing market is segmented into hospitals, clinics, and diagnostic laboratories & centers. The diagnostic laboratories & centers are anticipated to have the highest share in this market. The increased entry of outpatient tests for retroviral testing in early HIV-AIDS diagnosis is fueling the global market growth. High R&D investment and technological advancements also contribute to the diagnostic laboratories segment's revenue growth.
Recent Developments
- November 2020: The U.S. FDA approved Hologic Inc. for its Retroviral viral load monitoring assay. This has enabled the company to broaden its product offering in the HIV diagnostics market.
- May 2020: OraSure Technologies Inc. purchased UrSure Inc., a well-known laboratory testing company. This acquisition has expanded the company's HIV diagnostic product offerings.
Report Scope:
| Report Attributes | Report Details |
|---|---|
| Report Name | Retrovirus Testing Marke Size Report |
| Market Size in 2021 | USD 182.4 Million |
| Market Forecast in 2028 | USD 456.1 Million |
| Growth Rate | CAGR of 16.5% |
| Number of Pages | 188 |
| Forecast Units | Value (USD Billion), and Volume (Units) |
| Key Companies Covered | BGI Genomics, Abbott Diagnostics, Alere Inc, Hybribio Biotech, Roche Diagnostics Ltd, Tellgen Corporation, DiaSorin S.p.A., Dian Diagnostics, bioMerieux SA, Becton, Dickinson & Company, Xilong Scientific. |
| Segments Covered | By Type, By Contact Type, By Output And By Region. |
| Base Year | 2021 |
| Historical Year | 2018 to 2020 |
| Forecast Year | 2021 - 2028 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Retrovirus Testing Market: Regional Landscape
North America dominated the retrovirus testing market in 2021
Due to the increased awareness about retrovirus diagnosis, the existence of well-established healthcare infrastructure, and an increase in investments in activities of research & development, North America dominates the market for retrovirus testing. Moreover, according to HIV.gov statistics published in June 2021, nearly 2 million individuals in the United States have HIV. This suggests that the prevalence of HIV in the nation is high, which will increase demand for its diagnosing products and further propel the regional market. High investment in HIV rapid testing kits research and development contributes significantly to the regional market revenue growth.
Retrovirus Testing Market: Competitive Landscape
Some of the main competitors dominating the global retrovirus testing market include -
- BGI Genomics
- Abbott Diagnostics
- Alere Inc
- Hybribio Biotech
- Roche Diagnostics Ltd
- Tellgen Corporation
- DiaSorin S.p.A.
- Dian Diagnostics
- bioMerieux SA
- Becton
- Dickinson & Company
- Xilong Scientific.
Global Retrovirus Testing Market is segmented as follows:
By Test Type
- Infectivity Assay
- Product-Enhanced Reverse Transcriptase (PERT) Assay
- Co-Cultivation Assay
- Transmission Electron Microscopy
- Radio-Immune Assay
- Western Blot Analysis
- Immunofluorescence
- Serological Tests
By Technique Type
- High-Throughput Screening
- Enzyme Linked Immuno-Sorbent Assay (ELISA)
- Polymerase Chain Reaction (PCR)
By Sample Type
- Blood
- Serum
- Body Fluids
- Cells
By End User
- Hospitals
- Clinics
- Diagnostic Laboratories & Centers
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Adoption of in-vitro assay methods for retrovirus diagnosis is increasing due to an increase in the incidence of sexually transmissible diseases, as is an investment in research and development, boosting the global retrovirus testing market. The introduction of rapid and technologically sophisticated methods for detecting retroviral infections, rising awareness of diagnostic tests, and rising costs associated with retrovirus screening are anticipated to drive the global market for retrovirus testing during the forecast period.
According to the report, the global retrovirus testing market size was worth USD 182.4 million in 2021 and is estimated to grow to USD 456.1 million by 2028, with a compound annual growth rate (CAGR) of approximately 16.5 percent during the forecast period.
North America is anticipated to continue leading the retrovirus testing market throughout the projected period. The key factors driving the regional market growth are increased awareness about retrovirus diagnosis, well-established healthcare infrastructure, and increased research and development activities investments. Moreover, according to HIV.gov statistics published in June 2021, nearly 2 million individuals in the United States have HIV. This suggests that the prevalence of HIV in the nation is high, which will increase demand for its diagnosing products and further propel the regional market.
Some of the main competitors dominating the global retrovirus testing market include - BGI Genomics, Abbott Diagnostics, Alere Inc, Hybribio Biotech, Roche Diagnostics Ltd, Tellgen Corporation, DiaSorin S.p.A., Dian Diagnostics, bioMerieux SA, Becton, Dickinson & Company, Xilong Scientific.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed