Global Clinical Trial Supplies Market Is Likely To Grow At A CAGR Value Of Around 8.3% By 2028
03-May-2022 | Zion Market Research
The Clinical Trial Supplies Market was worth around USD 1,873.2 million in 2021 and is estimated to grow to about USD 3,022.4 million by 2028, with a compound annual growth rate (CAGR) of approximately 8.3 percent over the forecast period. The report analyzes the Clinical Trial Supplies Market drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the Clinical Trial Supplies Market.
A clinical trial is a study that assesses whether a medical strategy, treatment, or equipment is safe, effective, and helpful in humans. These investigations aid in determining which treatment techniques are most effective for specific disorders. A clinical trial is the most reliable source of information for making health-care decisions. Globalization, an increase in the number of clinical studies, and an increase in the number of chronic diseases in clinical trials are all projected to propel the industry forward. Rapid technical improvements in supply chain management are also likely to fuel market expansion. Clinical trial supplies make for a major portion of biopharmaceutical businesses' overall R&D spending, therefore the use of a supply chain management system is expanding due to rising demand to minimize R&D expenditure and boost operational efficiency. Virtual trials, which have been an underpinning technology since the Covid-19 epidemic, are progressively gaining popularity and are projected to experience significant development in the future years. The number of biologics and temperature-sensitive medications in clinical trials has increased dramatically. This percentage is projected to rise in the future, as biologics are becoming more popular due to fewer side effects when compared to traditional methods for storing temperature-sensitive medications. As a result, the demand for cold chain facilities is likely to expand as the number of biologics in clinical trials grows. The increased demand for biosimilars in both developing and developed economies is likely to boost cold chain supply, contributing to the clinical trial supplies market's growth throughout the forecast period. Clinical trials are now being undertaken primarily in developing countries. Clinical trials are increasingly being outsourced to Asia Pacific, Latin America, Central, and Eastern Europe, and the Middle East as the expense of conducting them rises and the recruiting of patients becomes more difficult, thus driving the regional market. The market is driven by illness variety in developing economies, which helps biopharmaceutical companies conduct clinical trials on uncommon diseases.
The Clinical Trial Supplies Market is segregated based on Services, phase, type, therapeutic area, and end-user. Based on Services, the global market is distinguished into Logistics & Distribution, Storage & retention, Packaging, labeling, and blinding, Manufacturing, Comparator sourcing, and Other services (solutions, ancillary supply). Based on phase, the global market is distinguished into Phase 1, Phase 2, Phase 3, Phase 4, and BA/ BE studies. Based on type, the global market is distinguished into Small molecules, Biologic drugs, and Medical devices. Based on therapeutic area, the global market is distinguished into Oncology, CNS and mental disorders, Cardiovascular diseases, Digestive disorders, Infectious diseases, Metabolic disorders, Immunology, Blood disorders, and Other therapeutic areas (respiratory disorders, dermatological disorders, rare diseases, ENT diseases, Nephrology). Based on end-user, the global market is distinguished into Pharmaceutical & Biotechnology Companies, Contract Research Organisations (CROs), and Medical Devices Companies.
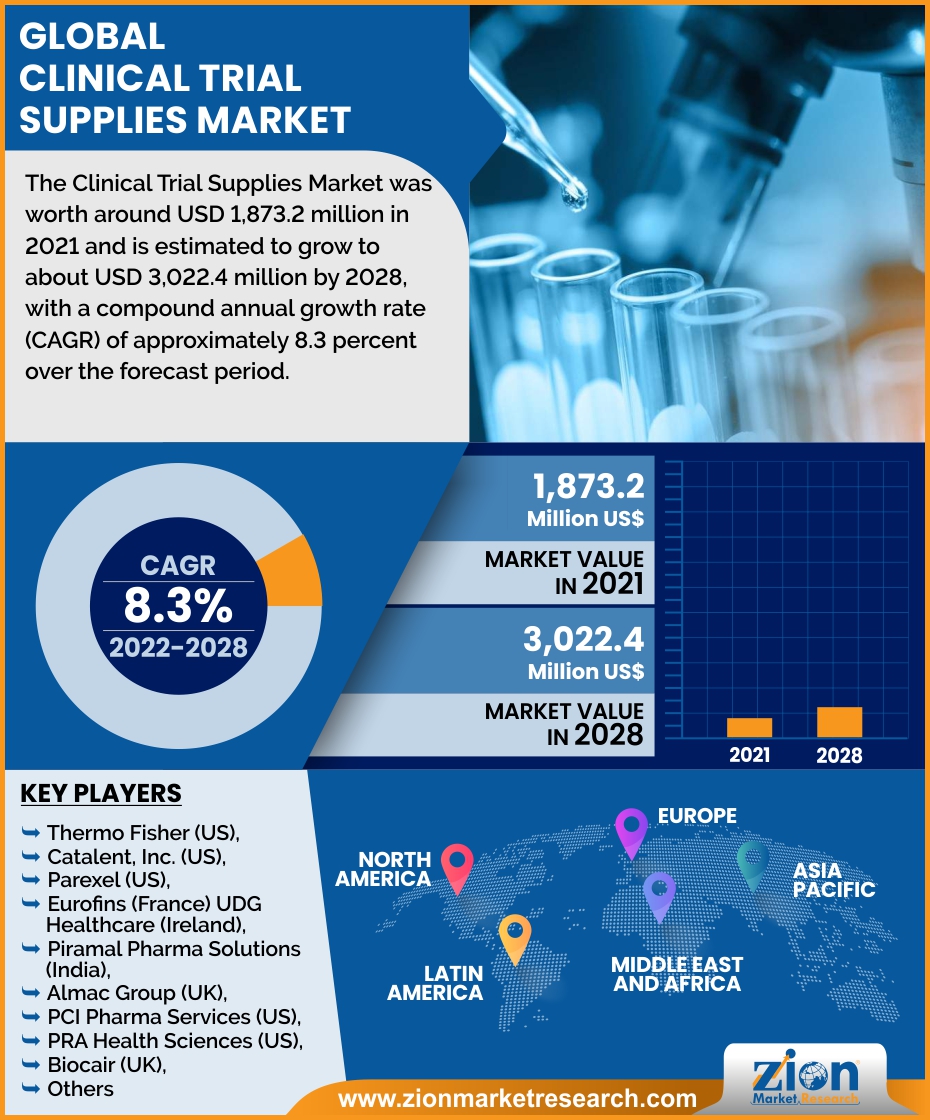
North America dominated the market and accounted for the largest revenue share in the forecast period due to the biggest number of clinical trials being undertaken in the area. The growing number of registered clinical trials in this region, as well as significant investments in clinical trial R&D, are primary factors driving the market's growth. The expanding number of healthcare companies conducting clinical trials in the region, supportive government laws, and the availability of cost-effective goods are all contributing to this expansion. Asia Pacific is predicted to be one of the market's fastest-growing regions. The market in the region is projected to be driven by the huge growth in clinical research. The presence of a broad set of patients who are easy to recruit is one of the key factors driving the rise of clinical research in the region.
Key players functioning in the Clinical Trial Supplies Market include Thermo Fisher (US), Catalent, Inc. (US), Parexel (US), Eurofins (France) UDG Healthcare (Ireland), Piramal Pharma Solutions (India), Almac Group (UK), PCI Pharma Services (US), PRA Health Sciences (US), Biocair (UK), Eurofins (France), Marken (US), Infosys (India), Liveo Research (India), Capsugel (a Lonza Group company) (Switzerland), SIRO Clinpharm (India), KLIFO A/S (Denmark), Clinigen (UK), Ancillare (US), N-SIDE (Belgium), ADAllen (UK), Rubicon (India), Durbin (UK), Recipharm (Sweden), Seveillar (India), Myonex (India).
Recent developments:
In 2021, Parexel and Veeva Systems (NYSE: VEEV) announced a strategic partnership to speed clinical trials by using technology and process innovation. To improve study efficiency and get new therapies to patients faster, the unique collaboration brings together the best of each company's experience across thousands of studies worldwide – Parexel as a leading clinical research organization (CRO) and Veeva as the technology innovator powering trials.
Browse the full “Clinical Trial Supplies Market By Services (Logistics & Distribution, Storage & retention, Packaging, labeling, and blinding, Manufacturing, Comparator sourcing, and Other services (solutions, ancillary supply)). By Phase (Phase 1, Phase 2, Phase 3, Phase 4, and BA/ BE studies). By Type (Small molecules, Biologic drugs, and Medical devices). By Therapeutic Area(Oncology, CNS and mental disorders, Cardiovascular diseases, Digestive disorders, Infectious diseases, Metabolic disorders, Immunology, Blood disorders, and Other therapeutic areas (respiratory disorders, dermatological disorders, rare diseases, ENT diseases, Nephrology)). By End-User (Pharmaceutical & Biotechnology Companies, Contract Research Organizations (CROs), and Medical Devices Companies)..” Report at https://www.zionmarketresearch.com/report/clinical-trial-supplies-market
Clinical Trial Supplies Market is segmented as follows:
By Services
- Logistics & distribution
- Storage & retention
- Packaging, labeling, and blinding
- Manufacturing
- Comparator sourcing
- Other services (solutions, ancillary supply)
By Phase
- Phase 1
- Phase 2
- Phase 3
- Phase 4
- BA/ BE studies
By Type
- Small molecules
- Biologic drugs
- Medical devices
By Therapeutic Area
- Oncology
- CNS and mental disorders
- Cardiovascular diseases
- Digestive disorders
- Infectious diseases
- Metabolic disorders
- Immunology
- Blood disorders
- Other therapeutic areas (respiratory disorders, dermatological disorders, rare diseases, ENT diseases, Nephrology)
By End-User
- Pharmaceutical & Biotechnology companies
- Contract Research Organisations (CROs)
- Medical Devices Companies
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
About Us:
Zion Market Research is an obligated company. We create futuristic, cutting-edge, informative reports ranging from industry reports, the company reports to country reports. We provide our clients not only with market statistics unveiled by avowed private publishers and public organizations but also with vogue and newest industry reports along with pre-eminent and niche company profiles. Our database of market research reports comprises a wide variety of reports from cardinal industries. Our database is been updated constantly in order to fulfill our clients with prompt and direct online access to our database. Keeping in mind the client’s needs, we have included expert insights on global industries, products, and market trends in this database. Last but not the least, we make it our duty to ensure the success of clients connected to us—after all—if you do well, a little of the light shines on us.
Contact Us:
Zion Market Research
244 Fifth Avenue, Suite N202
New York, 10001, United States
Tel: +49-322 210 92714
USA/Canada Toll-Free No.1-855-465-4651
Email: sales@zionmarketresearch.com
Website: https://www.zionmarketresearch.com
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed


