Active Implantable Medical Devices Market Size, Share, Growth, Trends, and Forecast 2028

Active Implantable Medical Devices Market By Product (Cardiac Pacemakers, Implantable Cardioverter Defibrillators (ICD), Nerve Simulators, Cochlear Implants, and Ventricular Assist Devices), By Application (Cardiovascular, Neurological, Hearing Impairment, and Others), By End-User (Hospitals, Specialty Clinics, Ambulatory Surgical Centers), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2022 - 2028
| Market Size in 2021 | Market Forecast in 2028 | CAGR (in %) | Base Year |
|---|---|---|---|
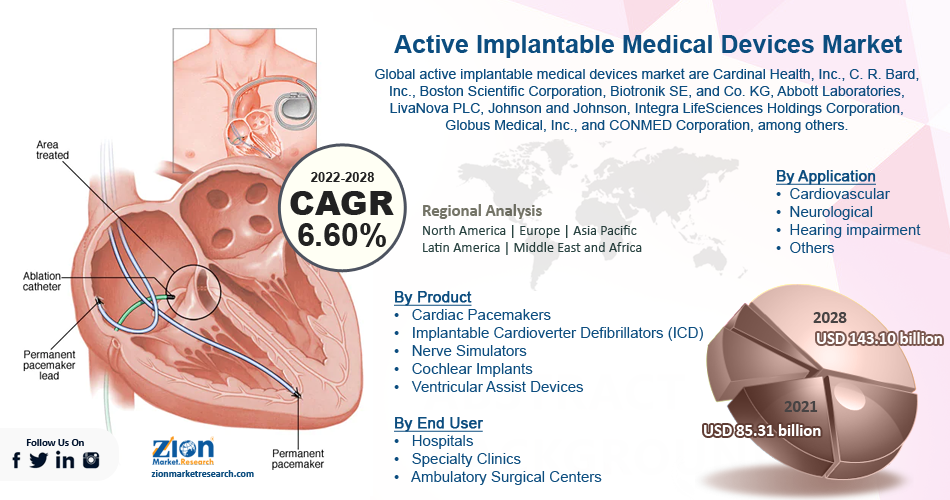
| USD 85.31 Billion | USD 143.10 Billion | 6.60% | 2021 |
Industry Prospective:
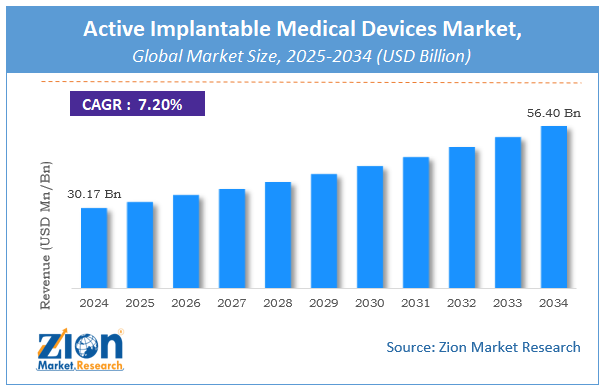
The global active implantable medical devices market was worth around USD 85.31 billion in 2021 and is estimated to grow to about USD 143.10 billion by 2028, with a compound annual growth rate (CAGR) of approximately 6.60 percent over the forecast period. The report analyzes the active implantable medical devices market’s drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the active implantable medical devices market.
Active Implantable Medical Devices Market: Overview
An Active Implantable Medical Device (AIMD) is a medical device that is implanted entirely or partially into the human body for therapeutic or diagnostic reasons and is meant to stay there. Neurostimulators, cochlear implants, ventricular assist systems & devices, infusion pumps, defibrillators, and pacemakers, are all examples of AIMDs.
Implantable medical devices are the type of medical devices that are partially or totally inserted into the human body with the help of medical intervention and are dedicated to remaining there after the procedure. They can support the damaged part, can replace the missing part of the human body or also modify the important function of the body.
COVID-19 Impact:
During the initial COVID-19 pandemic, there was a decrease in the number of patients admitted to hospitals for implant surgery. It was because individuals were afraid of becoming infected during the period of isolation and lockdown. Patients' admissions to hospitals were reduced in many locations, showing that several patients with moderate - to - severe disabilities skipped hospital visits. The net effect of COVID-19 on the implantable medical device sector has been reported to be adverse. The drop in demand for implant surgery was linked to this. Furthermore, since the number of patients admitted to hospitals for implant surgery has decreased, market participants have lowered their expenditure on raw materials and resources.
Active Implantable Medical Devices Market: Growth Drivers
Rising demand for active implantable medical devices is boosting the market growth
The need for active implantable devices has increased as the number of disorders and diseases has increased. These devices aid in the support or replacement of any injured bodily organs, as well as the enhancement and treatment of normal body functions. One of the most crucial aspects of patient care is an active implanted medical device. One of the key drivers of global active implantable medical device market growth is the increasing senior population. The functioning of numerous organs of the human body gradually deteriorates with age.
As a result, for movement and other help, humans must rely on a variety of medical gadgets. As a result, implantable devices are in high demand. Adults are affected by chronic ailments such as heart disease and arthritis, among others. One of the most frequent joint disorders in the United States is osteoarthritis (OA). It's also known as degenerative joint disease, and it affects the hands, hips, and knees the most. As a result of the country's rising elderly population, demand for orthopedic implants and reconstructive joint replacement is predicted to rise. All these factors are fostering market growth.
Active Implantable Medical Devices Market: Restraints
Device-related concerns to hinder the market growth.
Device-related concerns, such as network security and device failure, may impede market expansion. For instance, introducing a device into the human body may elicit an immune system from the surrounding tissues, resulting in performance deterioration, making long-term usage of these devices problematic. In addition to this, factors like less developed healthcare infrastructure in undeveloped countries, high cost of implants, and less awareness of medical devices in rural areas are restraining the market growth.
Active Implantable Medical Devices Market: Opportunities
Advancements in technology in the field of medical devices to boost the market during the forecast period.
Implantable medical devices are attracting more attention than ever before. Recently, the prospect of long-term and secure implantation in the human body without hazardous product release or device destruction by bodily fluids has appeared. Strengthening their portfolios and investing in technical improvements are two strategies used by device makers to increase revenue. Continuous advancements in electrode, packaging, microelectronics, and biomedical signal processing technology have fueled the development of active implantable medical devices (AIMDs) that transmit data and power through wireless telemetry. In addition to this, producers of AIMDs are making an investment in improving hydrolysis resistance, strength, implant stability, and radiolucency in MRIs, CT scans, & X-rays and the effects of ionizing radiation without imaging aberrations. All such factors are likely to create several opportunities for the global active implantable medical devices market growth during the forecast period.
Active Implantable Medical Devices Market: Challenges
Strict regulatory controls for medical devices to pose major challenge for the market growth
Active Implantable Medical Devices (AIMDs) are one of the most high-risk device types, requiring stringent regulatory oversight before reaching global markets. Both active implantable medical devices and their accessories are categorized as Class III, requiring the strictest regulatory control. Pre- and post-market, AIMDs are subject to stringent effective regulation. The MDR's regulatory requirements also apply to any accessories that are required for the device to function properly, including software programs, refill kits, programmers, leads, implant kits, controllers, and battery packs. Such strict regulations for AIMD pose a major challenge for the market expansion.
Active Implantable Medical Devices Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Active Implantable Medical Devices Market |
| Market Size in 2021 | USD USD 85.31 Billion |
| Market Forecast in 2028 | USD USD 143.10 Billion |
| Growth Rate | CAGR of 6.6% |
| Number of Pages | 180 |
| Key Companies Covered | Cardinal Health, Inc., C. R. Bard, Inc., Boston Scientific Corporation, Biotronik SE, Co. KG, Abbott Laboratories, LivaNova PLC, Johnson and Johnson, Integra LifeSciences Holdings Corporation, Globus Medical, Inc., CONMED Corporation, among others., |
| Segments Covered | By Product, By Application, By End User and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2021 |
| Historical Year | 2016 to 2020 |
| Forecast Year | 2022 - 2028 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Active Implantable Medical Devices Market: Segmentation
The global active implantable medical devices market is divided based on product, application, end-user, and region. By product, the global market is segregated into ventricular assist devices, cochlear implants, nerve stimulators, implantable cardioverter defibrillators (ICD), and cardiac pacemakers. Based on the application, the market is split into hearing impairment, neurological, cardiovascular, and others. The end-user segment is classified into ambulatory surgical centers, specialty clinics, and hospitals.
Recent Developments
- In February 2022, the FDA approved Abbott's CardioMEMSTM HF System which assists the patient with earlier-stage heart failure. Patients in the early stages of heart failure now have access to the CardioMEMSTM HF System, a tiny implanted sensor that can detect early warning signals of deteriorating heart failure.
- In April 2022, BIOTRONIK received FDA approval for Renamic Neo, its futuristic programmer for implanted cardiac rhythm management devices including pacemakers, ICDs, and implantable cardiac monitors.
Active Implantable Medical Devices Market: Regional Landscape
North America to hold the major share in the global market
North America is expected to hold the largest share of the global active implantable medical devices market, and this is expected to continue during the forecast period. The expanding demand for neurological and cardiovascular problems, as well as a rising focus on the development of sophisticated and novel products, are expected to propel the North American market forward in the approaching years. Furthermore, rising healthcare expenditures and increased consumer awareness of the availability of effective treatments are expected to fuel market expansion in the near future. In addition, Asia Pacific is expected to increase at a steady pace over the projection period. Increasing elderly populations and the rising frequency of chronic diseases are two key factors that are expected to propel the Asia Pacific market.
Active Implantable Medical Devices Market: Competitive Landscape
The predominant players that are successfully functioning in the global active implantable medical devices market are:
- Cardinal Health, Inc.
- C. R. Bard, Inc.
- Boston Scientific Corporation
- Biotronik SE
- Co. KG
- Abbott Laboratories
- LivaNova PLC
- Johnson and Johnson
- Integra LifeSciences Holdings Corporation
- Globus Medical, Inc.
- CONMED Corporation
- among others.
Global active implantable medical devices market is segmented as follows:
By Product
- Cardiac Pacemakers
- Implantable Cardioverter Defibrillators (ICD)
- Nerve Simulators
- Cochlear Implants
- Ventricular Assist Devices
By Application
- Cardiovascular
- Neurological
- Hearing impairment
- Others
By End User
- Hospitals
- Specialty Clinics
- Ambulatory Surgical Centers
By Region
-
North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Rising demand for active implantable medical devices is boosting the market growth. The need for active implantable devices has increased as the number of disorders and diseases has increased. These devices aid in the support or replacement of any injured bodily organs, as well as the enhancement and treatment of normal body functions. One of the most crucial aspects of patient care is an active implanted medical device.
According to the Zion Market Research report, the global active implantable medical devices market was worth about 85.31 (USD billion) in 2021 and is predicted to grow to around 143.10 (USD billion) by 2028, with a compound annual growth rate (CAGR) of around 6.60 percent.
North America is expected to hold the largest share of the global active implantable medical devices market, and this is expected to continue during the forecast period. The expanding demand for neurological and cardiovascular problems, as well as a rising focus on development and sophisticated and novel products, are expected to propel the North American market forward in the approaching years. Furthermore, rising healthcare expenditures and increased consumer awareness of the availability of effective treatments are expected to fuel market expansion in the near future.
The predominant players that are successfully functioning in the global active implantable medical devices market are Cardinal Health, Inc., C. R. Bard, Inc., Boston Scientific Corporation, Biotronik SE, and Co. KG, Abbott Laboratories, LivaNova PLC, Johnson and Johnson, Integra LifeSciences Holdings Corporation, Globus Medical, Inc., and CONMED Corporation, among others.
Choose License Type
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed