Biologics Safety Testing Market Size, Share, And Growth Report 2032

Biologics Safety Testing Market By Product (Services, Instruments and Reagents and Kits), By Test Type (Bioburden tests, Adventitious Agent Detection Tests, Residual Host Contamination Detection Tests, Cell Line Authentication and Characterization Tests, Sterility Tests, Endotoxin Tests and Other Test Types), By Application (Tissue and Tissue-related Products Testing, Cellular and Gene Therapy, Blood and Blood-related Products Testing, Vaccine and Therapeutics Development and Stem Cell Research), By Region - Global And Regional Industry Overview, market Intelligence, Comprehensive Analysis, Historical Data, And Forecasts 2024-2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 3.83 Billion | USD 10.82 Billion | 12.24% | 2023 |
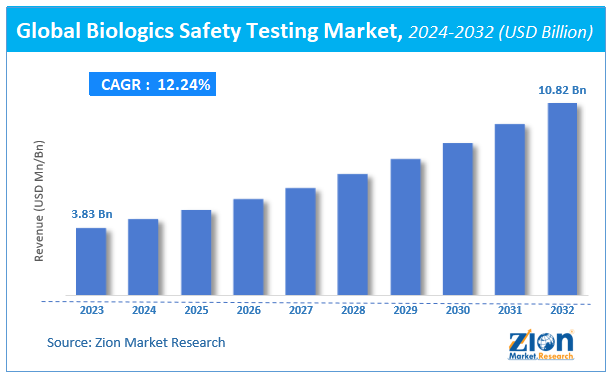
Biologics Safety Testing Market Size
The global Biologics Safety Testing market size was worth around USD 3.83 billion in 2023 and is predicted to grow to around USD 10.82 billion by 2032 with a compound annual growth rate (CAGR) of roughly 12.24% between 2024 and 2032.
The study provides historical data from 2018 to 2022 along with a forecast from 2024 to 2032 based on revenue (USD billion). The report covers a forecast and an analysis of the Biologics Safety Testing market on a global and regional level.
Biologics Safety Testing Market: Overview
Biologics products referred to products that are derives from biological sources. Biologics are used in animals and humans as medicines. These biologics include products derived from serum and blood, large polypeptides, vaccines, antitoxins, toxins, viruses, etc. Biologics safety is an important parameter for their use in animals and humans and also for regulatory approvals.
The demand for the biologics safety testing market is driven by increasing demand for biologics for treating various chronic diseases burden, increasing investments in life science research and development, rapid growth in new product launches, growth in pharmaceutical and biopharmaceutical research, and increasing prevalence of chronic disorders. However, stringent regulatory requirements and longer approval time may act as restraining factors for market growth. Increased preference for pharmaceutical outsourcing and emerging markets are expected to act as new growth opportunities for major market players in biologics safety testing.
The study includes drivers and restraints for the biologics safety testing market along with the impact they have on the demand over the forecast period. Additionally, the report includes the study of opportunities available in the biologics safety testing market on a global as well as regional level.
In order to give the users of this report a comprehensive view of the biologics safety testing market, we have included a competitive landscape and analysis of Porter’s Five Forces model for the market. The study encompasses a market attractiveness analysis, wherein all segments are benchmarked based on their market size, growth rate, and general attractiveness.
The report provides a company market share analysis in order to give a broader overview of the key players in the biologics safety testing market. In addition, the report also covers key strategic developments of the market including acquisitions & mergers, new product launches, agreements, partnerships, collaborations & joint ventures, research & development, and regional expansion of major participants involved in the biologics safety testing market on a global and regional basis.
Biologics Safety Testing Market: Segmentation
The study provides a crucial view on biologics safety testing by segmenting the market based on product, technology, test type, application, and region. All the segments of the biologics safety testing market have been analyzed based on present and future trends and the market is estimated from 2024 to 2032.
Based on product, the global biologics safety testing market is bifurcated into services, instruments, and reagents and kits. The reagents and kits segment held the largest market share in 2023 due to the repeated and consistent use of reagents and kits. The services segment will register rapid growth in the coming years with increasing demand for outsourcing services.
Based on test types, market is segmented into bioburden tests, adventitious agent detection tests, residual host contamination detection tests, cell line authentication, and characterization tests, sterility tests, endotoxin tests, and other test types. Endotoxin test type segment held the largest market share in 2023. sterility test type segment will witness the highest growth rate over the forecast period.
The application segment is divided into tissue and tissue-related product testing, cellular and gene therapy, blood and blood-related product testing, vaccine, and therapeutics development, and stem cell research. Vaccine and therapeutics development was the largest application segment for the biologics safety testing market in 2023. Increasing vaccine and therapeutic drug development activities by major biopharmaceutical and pharmaceutical companies have attributed to the largest market share.
Biologics Safety Testing Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Biologics Safety Testing Market |
| Market Size in 2023 | USD 3.83 Billion |
| Market Forecast in 2032 | USD 10.82 Billion |
| Growth Rate | CAGR of 12.24% |
| Number of Pages | 110 |
| Key Companies Covered | SGS SA, Merck KGaA, Charles River Laboratories, Lonza Group LTD., Toxikon Corporation, Pace Analytical Services Inc., Cytovance Biologics, Inc., Sartorius AG, Thermo Fisher Scientific Inc., and WuXi Apptec among others |
| Segments Covered | By product, By technology, By test type, By application and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Biologics Safety Testing Market: Regional Analysis
North America dominated the global biologics safety testing market in 2023. Increasing focus on the new vaccine and therapeutics development by pharmaceutical and biopharmaceutical companies, availability of funds, growing R&D investments, presence of developed R&D infrastructure, and technological advancements are factors driving the market in this region. Europe was the second largest regional market. High R&D spending, growing biologics development projects, government support for R&D, and the presence of developed infrastructure are some of the factors that boost the market growth in this region. Latin America is expected to witness considerable growth in the coming years. Asia Pacific region will exhibit the highest growth in the biologics safety testing market in the coming years. Increasing R&D spending, the focus of contract research organizations on capturing growth avenues in developing countries, increasing healthcare spending, and increasing chronic disease burden are factors propelling biologics safety testing market growth in the Asia Pacific. Lack of infrastructure and funds may attribute to slower growth in the Middle East and Africa.
Biologics Safety Testing Market: Competitive Players
Major players included in the report are:
- SGS SA
- Merck KGaA
- Charles River Laboratories
- Lonza Group LTD.
- Toxikon Corporation
- Pace Analytical Services Inc.
- Cytovance Biologics Inc.
- Sartorius AG
- Thermo Fisher Scientific Inc.
- WuXi Apptec
The Global Biologics Safety Testing Market is segmented as follows:
Global Biologics Safety Testing Market: By Product
- Reagents and Kits
- Services
- Instruments
Global Biologics Safety Testing Market: By Test Type
- Endotoxin Tests
- Sterility Tests
- Cell Line Authentication and Characterization Tests
- Residual Host Contamination Detection Tests
- Adventitious Agent Detection Tests
- Bioburden tests
- Other Tests
Global Biologics Safety Testing Market: By Application
- Vaccine and Therapeutics Development
- Blood and Blood-related Products Testing
- Cellular and Gene Therapy
- Tissue and Tissue-related Products Testing
- Stem Cell Research
Global Biologics Safety Testing Market: By Region
- North America
- The U.S.
- Europe
- UK
- France
- Germany
- Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- The Middle East and Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Biologics safety testing involves a series of evaluations designed to ensure the safety and efficacy of biologic products, such as vaccines, monoclonal antibodies, and gene therapies.
According to study, the Biologics Safety Testing Market size was worth around USD 3.83 billion in 2023 and is predicted to grow to around USD 10.82 billion by 2032.
The CAGR value of Biologics Safety Testing Market is expected to be around 12.24% during 2024-2032.
North America has been leading the Biologics Safety Testing Market and is anticipated to continue on the dominant position in the years to come.
The Biologics Safety Testing Market is led by players like SGS SA, Merck KGaA, Charles River Laboratories, Lonza Group LTD., Toxikon Corporation, Pace Analytical Services Inc., Cytovance Biologics Inc., Sartorius AG, Thermo Fisher Scientific Inc., and WuXi Apptec among others.
Choose License Type
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed