Bioprocess Validation Market Size, Share, Analysis, Trends, Growth, Forecasts, 2032

Bioprocess Validation Market - By Process Component (Bioreactors and Filter Element), By Test Type (Integrity Testing, Extractables & Leachable, and Microbiology Testing), and By End-User (Biotechnology & Pharmaceutical Companies, and CDMO) - Global Industry Perspective, Comprehensive Analysis, and Forecast, 2024-2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 374.07 Million | USD 1107.7 Million | 12.82% | 2023 |
Bioprocess Validation Industry Perspective
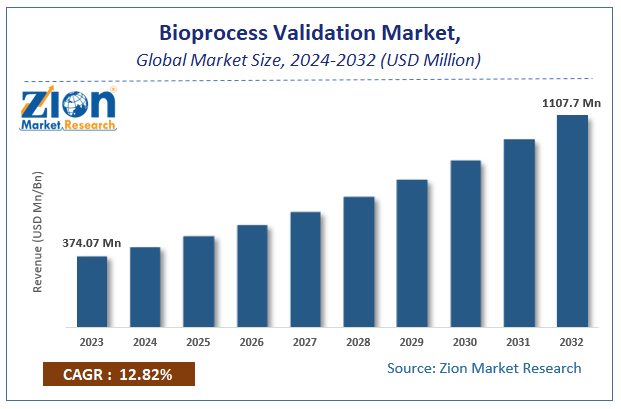
The global bioprocess validation market size accrued earnings worth approximately USD 374.07 Million in 2023 and is predicted to gain revenue of about USD 1107.7 Million by 2032, is set to record a CAGR of nearly 12.82% over the period from 2024 to 2032.
The report covers a forecast and an analysis of the bioprocess validation market on a global and regional level. The study provides historical data from 2018 to 2022 along with a forecast from 2024 to 2032 based on revenue (USD Billion). The study includes the drivers and restraints of the bioprocess validation market along with their impact on the demand over the forecast period. Additionally, the report includes the study of opportunities available in the patient handling device market on a global level.
Bioprocess Validation Market: Overview
The Bioprocess Validation Market involves the comprehensive testing and confirmation of bioprocesses to ensure they meet regulatory standards and produce consistent, high-quality products, particularly in the biopharmaceutical industry. Bioprocess validation is critical in the development and manufacturing of biologics, including vaccines, monoclonal antibodies, and other biopharmaceuticals. This market includes services and solutions related to equipment qualification, process validation, and cleaning validation, ensuring that the manufacturing processes are compliant with stringent regulatory requirements set by authorities like the FDA and EMA.
Bioprocess Validation Market: Growth Factors
The market is driven by factors such as the growing demand for biopharmaceuticals, increasing regulatory scrutiny, and the rising focus on quality assurance in the production of biologics. With the biopharmaceutical industry expanding rapidly due to advancements in biotechnology and the increasing prevalence of chronic diseases, the need for rigorous validation processes is greater than ever. Companies are investing heavily in bioprocess validation to minimize risks, ensure patient safety, and meet the stringent quality standards required in biopharmaceutical production.
The market is also influenced by technological advancements in validation processes, such as automation and data analytics, which enhance the efficiency and accuracy of validation activities. Additionally, the global expansion of biopharmaceutical manufacturing facilities, particularly in emerging markets, is expected to drive the demand for bioprocess validation services and solutions.
With the concept of the right product, at the right time, and at the right place becoming a buzzword in the biopharmaceutical industry, the market for bioprocess validation is anticipated to gain traction over the forecast period. Furthermore, new drugs are being launched in the biopharmaceutical sector and this will require bioprocess validation in the foreseeable future.
Apparently, the feasibility of operations & processes is a few of the challenges faced by both the pharmaceutical and biopharmaceutical industries. Furthermore, bioprocess validation assists in reducing the daunting process in both these industries. This, in turn, is anticipated to steer the expansion of the bioprocess validation industry over the forthcoming years.
Our report also provides the current developments taking place in the industry along with the competitive strategies adopted by the market players to expand their profit margins and try to consolidate their position in the market or optimize their market share in this fragmented industry. Let us discuss the initiatives taken by some of the reputed brands of the industry.
Merck KgaA
In January 2020, Merck KgaA, a renowned German-based pharmaceutical firm, announced that it will set up a biotech development unit in Switzerland. As per the company sources, the investments in the unit are likely to hit €250 million. Reportedly, the key objective of the firm is to interweave biologics development & creation. Furthermore, the CEO of the healthcare unit of Merck declared that the setting up of the biotech development center is related to the focus of the firm’s operations on healthcare activities.
For the record, the pharmaceutical giant is likely to complete the project by 2021 and have it authenticated by 2022. The center will cover 169,000 square feet of area and the new construction is expected to have the capacity to accommodate a cross-functional team of 250 employees. Furthermore, the center will include new technologies for reducing the lead & cycle times and offer the best process control, governance, and performance management practices to proficiently tackle the complexities of biomolecule drug development & delivery activities.
This, the strategic moves adopted by the reputed firms in the industry will have a major impact on the growth of the bioprocess validation industry over the years to come.
Bioprocess Validation Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Bioprocess Validation Market |
| Market Size in 2023 | USD 374.07 Million |
| Market Forecast in 2032 | USD 1107.7 Million |
| Growth Rate | CAGR of 12.82% |
| Number of Pages | 110 |
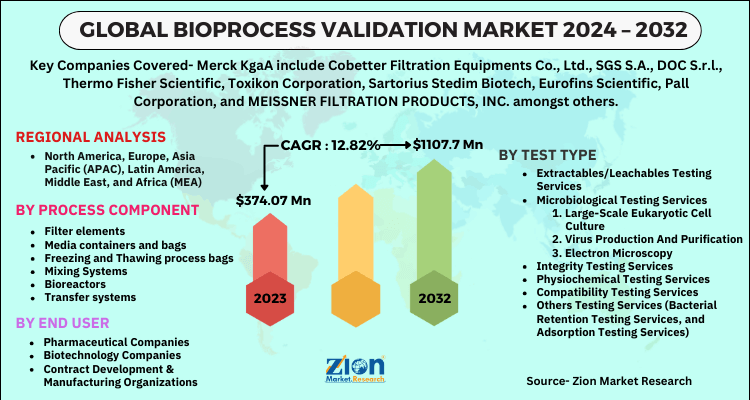
| Key Companies Covered | Merck KgaA include Cobetter Filtration Equipments Co., Ltd., SGS S.A., DOC S.r.l., Thermo Fisher Scientific, Toxikon Corporation, Sartorius Stedim Biotech, Eurofins Scientific, Pall Corporation, and MEISSNER FILTRATION PRODUCTS, INC. amongst others |
| Segments Covered | By Test Type, By Process Component, By End User and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Bioprocess Validation Market: Segmentation
The Bioprocess Validation Market can be segmented based on various factors, including the type of validation, stage of process, end user, and region. Below is a detailed overview of the segmentation:
1. By Type of Validation
- Process Validation: Ensures that the bioprocess consistently produces products that meet predetermined quality criteria. This includes process design, process qualification, and continued process verification.
- Cleaning Validation: Confirms that the cleaning processes effectively remove residues to a safe level, preventing contamination between production batches.
- Analytical Method Validation: Validates the analytical methods used to test the bioprocess output, ensuring accuracy, precision, specificity, and sensitivity of the tests.
- Equipment Validation: Involves qualifying the equipment used in the bioprocess to ensure it operates correctly and consistently.
- Computer System Validation: Ensures that computerized systems used in bioprocessing meet regulatory requirements and function as intended.
2. By Stage of Process
- Upstream Process Validation: Focuses on the initial stages of bioprocessing, including cell culture, fermentation, and harvest. It ensures that these processes are optimized and controlled to produce high-quality products.
- Downstream Process Validation: Involves validation of the processes used after initial production, such as purification, formulation, and filling. It ensures that these steps maintain the integrity and quality of the product.
3. By End User
- Pharmaceutical & Biotechnology Companies: The largest segment, as these companies are the primary users of bioprocess validation services to comply with regulatory standards.
- Contract Research Organizations (CROs): Provide outsourced validation services, often working with multiple clients in the pharmaceutical and biotech sectors.
- Academic & Research Institutes: Engage in bioprocess validation as part of research activities, particularly when developing new biopharmaceutical products.
- Government & Regulatory Bodies: Involved in overseeing and setting standards for validation processes within the industry.
4. By Region
- North America: The largest market due to the presence of a well-established biopharmaceutical industry, stringent regulatory requirements, and significant investments in R&D.
- Europe: Also a major market, driven by strong regulatory frameworks and a robust biotechnology sector.
- Asia-Pacific: Expected to witness the fastest growth, fueled by increasing biopharmaceutical manufacturing, growing healthcare infrastructure, and expanding investments in biotechnology.
- Latin America: Growth in this region is driven by the expanding pharmaceutical industry and increasing focus on biopharmaceuticals.
- Middle East & Africa: Emerging market with growing investments in healthcare and biopharmaceuticals, though currently smaller in scale compared to other regions.
5. By Service Provider
- In-House: Validation processes conducted internally by pharmaceutical and biotech companies.
- Outsourced: Validation services provided by external companies, such as CROs, which specialize in offering validation as a service.
This segmentation helps in understanding the different facets of the Bioprocess Validation Market and provides insights into the specific areas of growth and demand within the industry.
Bioprocess Validation Market: Competitive Space
Some of the leading players in the global market include
- Merck KgaA
- Cobetter Filtration Equipments Co. Ltd.
- SGS S.A.
- DOC S.r.l.
- Thermo Fisher Scientific
- Toxikon Corporation
- Sartorius Stedim Biotech
- Eurofins Scientific
- Pall Corporation
- MEISSNER FILTRATION PRODUCTS INC.
The global bioprocess validation market is segmented as follows:
By Test Type Analysis
-
Extractables/Leachables Testing Services
- Microbiological Testing Services
- Large-Scale Eukaryotic Cell Culture
- Virus Production And Purification
- Electron Microscopy
- Integrity Testing Services
- Physiochemical Testing Services
- Compatibility Testing Services
- Others Testing Services (Bacterial Retention Testing Services, and Adsorption Testing Services)
By Process Component Analysis
- Filter elements
- Media containers and bags
- Freezing and Thawing process bags
- Mixing Systems
- Bioreactors
- Transfer systems
- Others (Tubing, Connectors, Samplers)
By End User Analysis
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Development & Manufacturing Organizations
- Others (CROs, Research Laboratories and Institutes)
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Bioprocess validation is a critical procedure in the biopharmaceutical industry that ensures every step of the bioprocess, from development through production, consistently produces a product that meets defined quality standards.
The market is driven by factors such as the growing demand for biopharmaceuticals, increasing regulatory scrutiny, and the rising focus on quality assurance in the production of biologics.
According to a study, the global bioprocess validation Industry size was $374.07 Million in 2023 and is projected to reach $1107.7 Million by the end of 2032.
The global bioprocess validation market is expected to grow at a CAGR of 12.82% during the forecast period.
The major players active in the global bioprocess validation market are SGS S.A., Merck KGaA, Sartorius Stedim Biotech, Eurofins Scientific, Cobetter Filtration Equipments Co., Ltd., Pall Corporation, DOC S.r.l., Toxikon Corporation, Thermo Fisher Scientific, and MEISSNER FILTRATION PRODUCTS, INC.
Choose License Type
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed