Breakthrough Therapy (BT) Designation Market Size, Share, Trends, Growth and Forecast 2032

Breakthrough Therapy (BT) Designation Market - By Application (Oncology, Rare Diseases, Infectious Diseases, Pulmonary Diseases, Autoimmune Diseases, Neurological Disorders, Others), And By Region - Global Industry Perspective, Comprehensive Analysis, and Forecast, 2024 - 2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 130.78 Billion | USD 529.08 Billion | 16.8% | 2023 |
Breakthrough Therapy (BT) Designation Market Insights
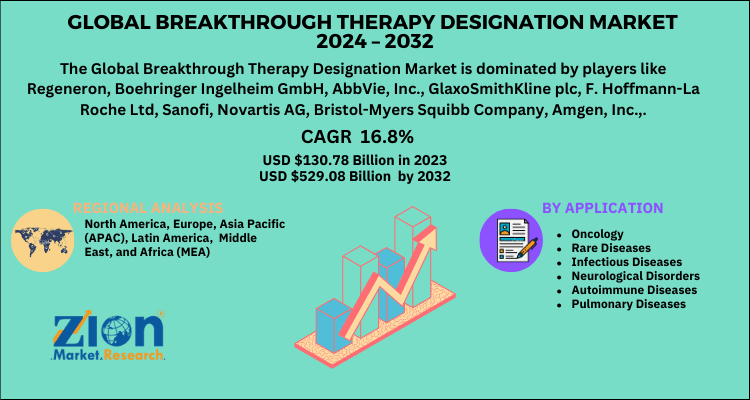
According to a report from Zion Market Research, the global Breakthrough Therapy (BT) Designation Market was valued at USD 130.78 Billion in 2023 and is projected to hit USD 529.08 Billion by 2032, with a compound annual growth rate (CAGR) of 16.8% during the forecast period 2024-2032.
This report explores market strengths, weakness, opportunities, and threats. It also provides valuable insights into the market's growth drivers, challenges, and the future prospects that may emerge in the Breakthrough Therapy (BT) Designation industry over the next decade.
Breakthrough Therapy (BT) Designation Market: Overview
Breakthrough Therapy (BT) Designation is a procedure that is designed for development and reviewing of medicines that assist in treatment of severe medical conditions. Furthermore, medicine that receives BT designation is eligible for all kinds of fast track designation features, extensive guidance on effective drug development project, and organizational commitment that includes senior managers. Reportedly, U.S. FDA has recommended use of breakthrough therapy designation for pharmaceutical or drug manufacturing firms. Massive requirement for effective cancer therapy from among various cancer treatments has resulted in elevation in the market demand.
Breakthrough Therapy (BT) Designation Market?: Growth Drivers
Inflating consumption of orphan medicines and large-scale acceptance of BT status in drugs will embellish the market expansion over the years ahead. Need for providing effective drug treatment to patients suffering from life-threatening ailments and necessity of bringing improvement in disease condition through determining of effective drug therapies will boost the market trends. In addition to this, surge in healthcare spending in both emerging economies and developed countries will prompt the market size over the forecast timeline. Citing an instance, there has been a prominent surge in the number of medicines getting BT status in the countries such as the U.S.
Furthermore, rise in the infectious diseases and an increase in the pandemics like Swine Flu, COVID-19, Ebola, and Bird Flu will create new growth avenues for the breakthrough therapy (BT) designation industry in the years ahead. Nonetheless, huge costs of the BT-designated medicines will restrict business growth. Apparently, easy availability of generic medicines will put more brakes on the industry expansion in the years ahead.
Breakthrough Therapy (BT) Designation Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Breakthrough Therapy (BT) Designation Market |
| Market Size in 2023 | USD 130.78 Billion |
| Market Forecast in 2032 | USD 529.08 Billion |
| Growth Rate | CAGR of 16.8% |
| Number of Pages | 110 |
| Key Companies Covered | Regeneron; Boehringer Ingelheim GmbH; AbbVie, Inc.; GlaxoSmithKline plc; F. Hoffmann-La Roche Ltd; Sanofi; Novartis AG; Bristol-Myers Squibb Company; Amgen, Inc.; Acadia Pharmaceuticals, Inc.; Pfizer, Inc.; AstraZeneca; Gilead; Janssen Global Services, LLC; and Eli Lilly and Company |
| Segments Covered | By Application And By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Breakthrough Therapy (BT) Designation Market: Regional Insights
North America To Establish Dominant Position In Global Market By 2032
The expansion of the industry in North American sub-continent over the assessment period is attributed to favorable intellectual property legislations rewarding breakthroughs through data protection and copyright security. In addition to this, easy access to advanced healthcare facilities and surge in chronic disorders will promulgate market penetration over the forthcoming years.
Breakthrough Therapy (BT) Designation Market: Competitive Space
Key players leveraging the market growth are -
- Regeneron
- Boehringer Ingelheim GmbH
- AbbVie, Inc.
- GlaxoSmithKline plc
- F. Hoffmann-La Roche Ltd
- Sanofi
- Novartis AG
- Bristol-Myers Squibb Company
- Amgen, Inc.
- Acadia Pharmaceuticals, Inc.
- Pfizer, Inc.
- AstraZeneca
- Gilead
- Janssen Global Services, LLC
- Eli Lilly and Company.
The global breakthrough therapy (BT) designation market is segmented as follows:
By Application:
- Oncology
- Rare Diseases
- Infectious Diseases
- Neurological Disorders
- Autoimmune Diseases
- Pulmonary Diseases
- Others
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Inflating consumption of orphan medicines and large-scale acceptance of BT status in drugs will embellish the market expansion over the years ahead. Need for providing effective drug treatment to patients suffering from life-threatening ailments and necessity of bringing improvement in disease condition through determining of effective drug therapies will boost the market trends. In addition to this, surge in the healthcare spending in both emerging economies and developed countries will prompt the market size over the forecast timeline.
According to a report from Zion Market Research, the global Breakthrough Therapy (BT) Designation Market was valued at USD 130.78 Billion in 2023 and is projected to hit USD 529.08 Billion by 2032, with a compound annual growth rate (CAGR) of 16.8% during the forecast period 2024-2032
North America is likely to make noteworthy contributions towards overall market revenue. The regional market growth over 2019-2025 can be credited to favorable intellectual property legislations rewarding breakthroughs through data protection and copyright security. In addition to this, easy access to advanced healthcare facilities and surge in the chronic disorders will promulgate the market penetration over the forthcoming years.
The key players profiled in the report include Regeneron; Boehringer Ingelheim GmbH; AbbVie, Inc.; GlaxoSmithKline plc; F. Hoffmann-La Roche Ltd; Sanofi; Novartis AG; Bristol-Myers Squibb Company; Amgen, Inc.; Acadia Pharmaceuticals, Inc.; Pfizer, Inc.; AstraZeneca; Gilead; Janssen Global Services, LLC; and Eli Lilly and Company.
Choose License Type
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed

-designation-market-size.png)