Clinical Trials Market Size, Share, Growth Report 2032

Clinical Trials Market By Phase (Phase I, Phase II, Phase III, Phase IV) By Study Design (Observational, Interventional, Expanded Access) By Indication (Gastrointestinal, Cardiovascular, Autoimmune/Inflammation, Pain Management, CNS Condition, Cancer, Mental Disorders, Oncology, Diabetes, Obesity, Others), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2023 - 2030
| Market Size in 2022 | Market Forecast in 2030 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 53.92 Billion | USD 85.01 Billion | 5.93% | 2022 |
Clinical Trials Industry Perspective:
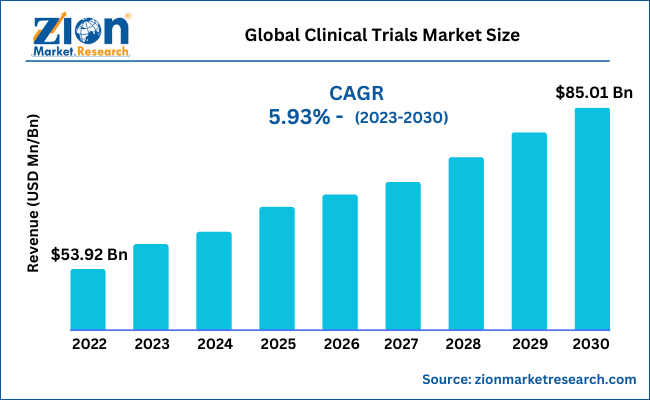
The global clinical trials market size is set for rapid growth over the forecast period. In terms of revenue, the global clinical trials market accounted for USD 53.92 Billion in 2022 and is expected to reach USD 85.01 Billion by 2030, growing at a CAGR of 5.93% during the forecast period.
The market report offers quantitative and qualitative insights into the key drivers, opportunities, constraints, and challenges impacting the global clinical trials industry.
Clinical Trials Market: Overview
Clinical trials are research experiments carried out in individuals aimed at testing a medical, behavioral, or surgical intervention. They are the key platform for researchers to find out if a new medication is safe and successful in humans, such as a new medicine, medical device, or diet. A clinical study is also used to assess whether new treatments are more successful and/or have fewer adverse side effects than traditional therapies.
Other clinical trials are exploring ways of detecting disease early, even before symptoms appear. It also researches ways to stop a health condition. A clinical study can also look at how people coping with a life-threatening illness or a chronic health condition can make life easier. Often, clinical trials research the involvement of caregivers or support groups.
Before the United States Food and Drug Administration (FDA) approves the launch of a clinical trial, in which scientists conduct experimental tests and animal trials to assess the safety and effectiveness of possible treatment. The FDA gives permission for the intervention to be studied in humans if these studies produce favorable results.
Key Insights
- As per the analysis shared by our research analyst, the global clinical trials market is expected to grow annually at a promising CAGR of around 5.93% during the forecast period 2023-2030.
- In terms of revenue, the global clinical trials market size was valued at around USD 53.92 billion in 2022 and is expected to reach USD 85.01 billion by 2030.
- The clinical trials market is experiencing significant growth due to the increasing prevalence of chronic & infectious diseases, demand for personalized medicine, and advancements in technology.
- Based on phase, the phase 3 segment held the largest market share in 2022.
- Based on study design, the interventional segment held the dominating market share in 2022.
- Based on region, the North American region held the largest market share in 2022.
Clinical Trials Market: Growth Factors
Increase in the prevalence of chronic diseases and surge in demand for personalized medicine drive the market growth
The global clinical trials market is experiencing significant growth due to the increasing prevalence of chronic and infectious diseases, demand for personalized medicine, and advancements in technology. The market is further driven by the rising investment by pharmaceutical and biotechnology companies and favorable government initiatives to support clinical research and development. Moreover, the increasing adoption of virtual and decentralized clinical trials is fueling market growth, as they enable faster recruitment, reduce trial costs, and offer improved patient experiences. Additionally, the emergence of contract research organizations (CROs) is contributing to market expansion, as they provide cost-effective solutions to drug developers.
Furthermore, the use of artificial intelligence, machine learning, and big data analytics is expected to transform the clinical trials landscape by improving trial efficiency and accuracy. Thus, the clinical trials market is expected to grow significantly in the coming years.
Clinical Trials Market: Restraints
Extortionate cost of conducting trials to hamper the growth of the industry
The clinical trials industry also faces several restraints, including the high cost associated with conducting trials and complex regulatory requirements. Additionally, the COVID-19 pandemic has disrupted clinical trials, leading to delays, cancellations, and reduced enrollment. Moreover, the lack of patient diversity in clinical trials is a significant challenge, as it limits the generalizability of trial results to broader populations. Furthermore, ethical concerns and the need for informed consent from participants pose additional challenges to the market. Finally, there is a need for increased collaboration between stakeholders, including sponsors, CROs, regulators, and patients, to overcome these challenges and ensure the success of clinical trials.
Clinical Trials Market: Opportunities
Increase in the development of new technologies create ample opportunities for the industry
Despite the challenges, the global clinical trials market presents several opportunities for growth. One of the significant opportunities is the increasing focus on precision medicine, which requires personalized and targeted therapies based on patient-specific characteristics. This approach necessitates the development of more sophisticated and specialized clinical trials, providing opportunities for CROs and other service providers.
Moreover, the growing interest in rare diseases and orphan drugs presents significant opportunities for clinical trials, as these areas require specialized expertise and resources. Furthermore, the emergence of real-world evidence (RWE) and patient-generated health data (PGHD) offers new avenues for clinical research, improving trial design and outcomes.
The adoption of new technologies, such as telemedicine and wearables, is also expected to expand the reach of clinical trials and increase patient participation. Furthermore, collaborations and partnerships between pharmaceutical companies, CROs, and academic institutions can help drive innovation and improve the efficiency of clinical trials.
Clinical Trials Market: Challenges
Shortage of skilled professionals to act as a challenge for the industry
The clinical trials industry faces several challenges, including complex regulatory requirements, the shortage of skilled professionals, disruptions caused by the COVID-19 pandemic, and a lack of patient diversity. Additionally, ethical concerns and the need for informed consent from participants pose significant challenges to the market. Overcoming these challenges requires increased collaboration between stakeholders, the adoption of new technologies, and innovative trial designs that promote patient participation and diversity.
Impact due to the outbreak of COVID-19
The emergence of a global coronavirus-induced pandemic is also driving the demand. Lives, populations, firms, and industries around the world are threatened by the rapidly emerging threat due to the outbreak of COVID-19. The pandemic has also adversely affected the current clinical trial ecosystem. For different clinical fields, it has influenced many ongoing trials. To resolve this, however, researchers are vigorously working on developing innovative therapeutics and COVID-19 vaccines which is encouraging market development.
The latest pandemic is changing the way ongoing or upcoming clinical trials are performed. For example, regulatory agencies such as the European Medicines Agency (EMA), the U.S. Food and Drug Administration (FDA), China’s National Medical Products Administration, and the National Institutes of Health (NIH) among others have released recommendations relating to the execution of coronavirus outbreak trials, which are completely endorsed by the inclusion of virtual services.
The existing worldwide situation and the need to come up with alternatives for treatment have also contributed to the fast-track of clinical trials. The favorable support of the government boosts the growth of the sector. In order to find successful therapy against COVID-19, the World Health Organization (WHO) initiated "Solidarity," an international clinical trial. Test their efficacy against Coronavirus, which involves reviewing four treatment options against the standard of care. In May, the WHO also announced an international partnership to establish multiple candidate vaccines simultaneously to combat the risk of coronavirus disease, naming this initiative the Solidarity vaccine trial.
Clinical Trials Market: Segmentation
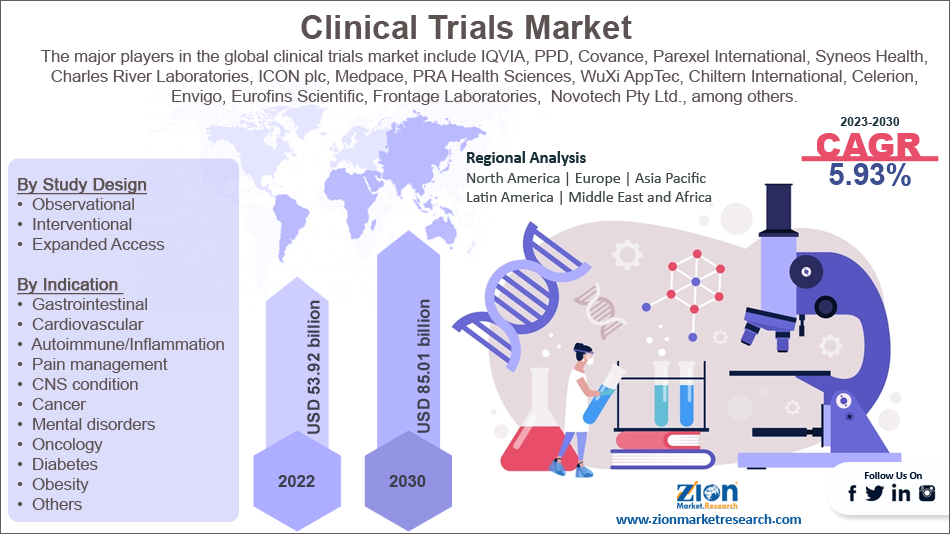
The global clinical trials market is segmented based on phase, study design, indication, and region.
Based on phase, the market bifurcated into Phase I, Phase II, Phase III, and Phase IV. The Phase III segment held the largest market share in 2022 and is further predicted to grow rapidly at a notable CAGR during the forecast period. The main reason for the growth of this segment is its critical role in drug development. Phase III trials are designed to compare the safety and efficacy of new treatments to existing therapies, providing important data for regulatory approval and market access.
The increasing prevalence of chronic and infectious diseases and demand for personalized medicine is driving the need for new and innovative treatments, leading to a surge in Phase III trials. Moreover, the emergence of real-world evidence and patient-generated health data is expected to transform the Phase III landscape, enabling more efficient and effective trials.
Based on study design, the clinical trials industry is bifurcated into observational, interventional, and expanded access. The interventional design segment held the dominating market share in 2022 and is further projected to grow rapidly at a significant CAGR during the forecast period.
The interventional design segment of the clinical trials market is expected to experience significant growth due to the increasing demand for innovative and effective treatments. Interventional trials involve the use of a new treatment or drug and are designed to evaluate its safety, efficacy, and side effects. These trials play a crucial role in the development of new therapies and are essential for gaining regulatory approval and market access. The rising prevalence of chronic & infectious diseases and demand for personalized medicine is driving the need for new & innovative treatments, leading to a surge in interventional trials. Moreover, advancements in technology and data analytics are expected to transform interventional trial design, making them more efficient and effective.
Based on indication, the market is segmented into gastrointestinal, cardiovascular, autoimmune/inflammation, pain management, CNS condition, cancer, mental disorders, oncology, diabetes, obesity, and others. The oncology segment held the dominating market share in 2022 and is projected to cite a remarkable CAGR during the forecast period.
Recent Developments
- In October 2021, Novartis announced the completion of its Phase III clinical trial for a new sickle cell disease treatment. The trial showed a significant reduction in the rate of sickle cell-related pain crises compared to the placebo, and the company plans to submit the drug for regulatory approval in 2022.
- In June 2021, AstraZeneca announced positive results from its Phase III clinical trial for a new lung cancer treatment. The trial showed a significant improvement in progression-free survival compared to the placebo, and the company plans to submit the drug for regulatory approval.
- In September 2021, Pfizer and BioNTech announced the completion of a Phase II/III trial for their COVID-19 vaccine in children aged 5 to 11. The trial showed a favorable safety profile and robust antibody responses, and the companies plan to seek regulatory authorization for the vaccine in this age group.
Clinical Trials Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Clinical Trials Market Research Report |
| Market Size in 2022 | USD 53.92 Billion |
| Market Forecast in 2030 | USD 85.01 Billion |
| Growth Rate | CAGR of 5.93% |
| Number of Pages | 215 |
| Key Companies Covered | IQVIA, PPD, Covance, Parexel International, Syneos Health, Charles River Laboratories, ICON plc, Medpace, PRA Health Sciences, WuXi AppTec, Chiltern International, Celerion, Envigo, Eurofins Scientific, Frontage Laboratories, Laboratory Corporation of America Holdings (LabCorp), Novotech Pty Ltd., Pharmaron, Pharmaceutical Product Development, LLC (PPD), and QuintilesIMS (now IQVIA) among others. |
| Segments Covered | By Phase, By Study Design, By Indication, And By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2022 |
| Historical Year | 2017 to 2021 |
| Forecast Year | 2023 - 2030 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Clinical Trials Market: Regional Analysis
North America region to dominate the global market during the forecast period
Based on region, the North American region held the dominating clinical trials market share in 2022 and is further predicted to grow at an exponential CAGR during the forecast period. North America is a major player in the clinical trials market, with the United States accounting for the majority of the region's market share. The growth of the market in North America is driven by several factors, including the increasing prevalence of chronic & infectious diseases, the demand for personalized medicine, and the presence of a well-established pharmaceutical industry.
Furthermore, the region has a favorable regulatory environment, with the FDA playing a crucial role in the approval and oversight of clinical trials. The COVID-19 pandemic has also spurred growth in the North American clinical trials market, with a surge in trials for vaccines and treatments. Therefore, the North American clinical trials market is expected to continue growing due to the region's strong healthcare infrastructure, favorable regulatory environment, and increasing demand for innovative treatments.
Clinical Trials Market: Competitive Players
The major players in the global clinical trials market include:
- IQVIA
- PPD
- Covance
- Parexel International
- Syneos Health
- Charles River Laboratories
- ICON plc
- Medpace
- PRA Health Sciences
- WuXi AppTec
- Chiltern International
- Celerion
- Envigo
- Eurofins Scientific
- Frontage Laboratories
- Laboratory Corporation of America Holdings (LabCorp)
- Novotech Pty Ltd.
- Pharmaron
- Pharmaceutical Product Development LLC (PPD)
- QuintilesIMS (now IQVIA)
- Among Others.
The report segment of the global clinical trials market is as follows:
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design
- Observational
- Interventional
- Expanded Access
By Indication
- Gastrointestinal
- Cardiovascular
- Autoimmune/Inflammation
- Pain management
- CNS condition
- Cancer
- Mental disorders
- Oncology
- Diabetes
- Obesity
- Others
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Clinical trials are research experiments carried out in individuals aimed at testing a medical, behavioral, or surgical intervention. They are the key platform for researchers to find out if a new medication is safe and successful in humans, such as a new medicine, medical device, or diet. A clinical study is also used to assess whether new treatments are more successful and/or have fewer adverse side effects than traditional therapies.
The global clinical trials market is predicted to increase at a CAGR of 5.93% during the forecast period.
The global clinical trials market was worth around USD 53.92 billion in 2022 and is expected to reach USD 85.01 billion by 2030.
The global clinical trials market is being driven by several factors, including the increasing prevalence of chronic and infectious diseases, growing demand for personalized medicine, advancements in technology, rising investment by pharmaceutical and biotechnology companies, and favorable government initiatives to support clinical research and development.
North America held a notable share in the global clinical trials market in 2022 and is likely to grow the fastest during the forecast period. The growth of the clinical trials market in North America is due to the presence of a large number of pharmaceutical and biotech companies, favorable regulatory framework, increasing investment in R&D activities, rising prevalence of chronic diseases, and growing demand for personalized medicine. The use of advanced technologies and the availability of skilled professionals further fuel market growth.
Some of the major companies operating in the clinical trials market include IQVIA, PPD, Covance, Parexel International, Syneos Health, Charles River Laboratories, ICON plc, Medpace, PRA Health Sciences, WuXi AppTec, Chiltern International, Celerion, Envigo, Eurofins Scientific, Frontage Laboratories, Laboratory Corporation of America Holdings (LabCorp), Novotech Pty Ltd., Pharmaron, Pharmaceutical Product Development, LLC (PPD), and QuintilesIMS (now IQVIA) among others.
Choose License Type
List of Contents
Industry Perspective: OverviewKey Insights Growth FactorsRestraintsOpportunitiesChallengesImpact due to the outbreak of COVID-19SegmentationRecent Developments Market:Report ScopeRegional AnalysisCompetitive PlayersThe report segment of the global clinical trials market is as follows:RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed