Deuterated Drugs Market Size, Share, Trends, Growth and Forecast 2032

Deuterated Drugs Market By Type (Austedo, Zepsun and Others), By Application (Cancer, Huntington's Disease (HD), Tardive Dyskinesia (TD) and Others), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2024 - 2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 341.05 Million | USD 908.43 Million | 11.50% | 2023 |
Deuterated Drugs Industry Prospective:
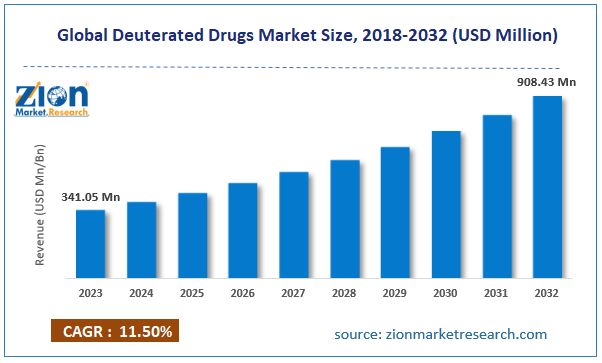
The global deuterated drugs market size was worth around USD 341.05 million in 2023 and is predicted to grow to around USD 908.43 million by 2032 with a compound annual growth rate (CAGR) of roughly 11.50% between 2024 and 2032.
Deuterated Drugs Market: Overview
A deuterated medication is a small molecular structure that has had one or more of its hydrogen atoms swapped out for the stable, heavier isotope deuterium. Deuteration has an impact on the drug's pharmacokinetic characteristics, including metabolism. Deuteration enhances the stability, safety, and tolerance of the medicine by decreasing its metabolism and strengthening its chemical bonds. Drugs that are deuterated are less poisonous, more effective, and have a longer half-life. Deuterated medications are used to treat tardive dyskinesia, cancer, and chorea (an involuntary movement disorder) linked to Huntington's disease. The first deuterated medicine molecule, deutetrabenazine, an analog of the previous medication tetrabenazine, has been approved for commercialization by the FDA. To treat chorea, one uses tetrabenazine.
Key Insights
- As per the analysis shared by our research analyst, the global deuterated drugs market is estimated to grow annually at a CAGR of around 11.50% over the forecast period (2024-2032).
- In terms of revenue, the global deuterated drugs market size was valued at around USD 341.05 million in 2023 and is projected to reach USD 908.43 million, by 2032.
- The growing clinical trial for deuterated drugs in several rare diseases such as Huntington's is expected to drive the market growth.
- Based on the type, the Austedo segment is expected to dominate the market during the forecast period.
- Based on the application, the Huntington's Disease (HD) segment is expected to hold the dominant position in the deuterated drugs industry.
- Based on region, North America is expected to dominate the market during the forecast period.
 Request Free Sample
Request Free Sample
Deuterated Drugs Market: Growth Drivers
Enhanced drug safety and growing therapeutic application drive the market growth
When it comes to side effects and toxicity, deuterated medications may be less than their non-deuterated equivalents. Deuteration can potentially reduce the production of hazardous metabolites or increase the drug's selectivity for its intended target by altering its metabolism and distribution, which could result in a safer therapeutic profile. Deuteration also makes it possible to create medications with enhanced qualities for a variety of medical purposes. This covers conditions that have difficult treatment requirements or for which the safety, effectiveness, or dose schedules of currently available drugs are restricted.
Deuterated Drugs Market: Restraints
Safety and regulatory concerns hinder market growth
While the usage of deuterated medications may raise safety concerns, they may also offer better pharmacological qualities. Comprehensive examination through preclinical and clinical investigations is necessary to determine the long-term consequences of deuterium incorporation on drug metabolism, toxicity, and off-target effects. Safety concerns may limit the market acceptability of deuterated medications or cause delays in regulatory authorization. Furthermore, getting regulatory approval for deuterated pharmaceuticals may provide extra difficulties, even in the face of regulatory support for novel approaches to drug development. Regulatory bodies can need a lot of evidence to prove the quality, safety, and efficacy of deuterated medications, especially if they differ from traditional drug development methods.
Deuterated Drugs Market: Opportunities
Rising partnership offers a lucrative opportunity for market growth
The increasing partnership among the key market players is expected to offer a lucrative opportunity for market growth during the forecast period. For instance, in February 2024, a partnership was announced between Teva Pharmaceutical Investments Singapore Pte Ltd, a subsidiary of Teva Pharmaceutical Industries Ltd., and Jiangsu Nhwa Pharmaceutical Co., Ltd. for the marketing and distribution of Teva's AUSTEDO (deutetrabenazine), which is used to treat neurodegenerative and movement disorders in adults, including tardive dyskinesia (TD) and chorea associated with Huntington's disease (HD). Through this cooperation, Teva's AUSTEDO will be more widely available to patients, capitalizing on Nhwa's prominence in China's neuro-psychiatric healthcare market.
Deuterated Drugs Market: Challenges
Growing competition poses a major challenge to market expansion
Many businesses are fighting for market share in the very competitive pharmaceutical sector. Biologics, gene therapies, and RNA-based medicines are a few of the cutting-edge pharmacological modalities that compete with deuterated pharmaceuticals. The market's competition may restrict the uptake and financial viability of deuterated medications.
Deuterated Drugs Market: Segmentation
The global deuterated drugs industry is segmented based on type, application and region.
Based on the type, the global deuterated drugs market is bifurcated into Austedo, Zepsun and Others. The Austedo segment is expected to dominate the market during the forecast period. The market for deuterated medications has grown as a result of Austedo's success, especially in the neurology sector. Since Austedo's approval, people, pharmaceutical companies, and healthcare professionals have become more knowledgeable about and interested in deuterated medications. In addition, the growing product approvals are one of the prominent factors that propel the segment expansion. For instance, in February 2023, The FDA approved AUSTEDOXR (deutetrabenazine) extended-release tablets, a novel once-daily formulation recommended in adults for tardive dyskinesia (TD) and chorea associated with Huntington's disease (HD). Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd., made this announcement. An extra version of the currently available twice-daily AUSTEDO is called AUSTEDO XR. The twice-daily version of AUSTEDO XR has been demonstrated to be therapeutically equal to the once-daily formulation, offering an additional efficient therapy option. Three extended-release tablet strengths (6 mg, 12 mg, and 24 mg) will be offered; it can be taken with or without food. When compared to the twice-daily formulation, the new tablet strengths offer an updated regimen that might cause patients to take fewer pills overall.
Based on the application, the global deuterated drugs industry is segmented into Cancer, Huntington's Disease (HD), Tardive Dyskinesia (TD) and Others. The Huntington's Disease (HD) segment is expected to hold the dominant position in the deuterated drugs industry. The segment growth is attributed to the rising prevalence of Huntington's Disease (HD). Moreover, preclinical and clinical research on deuterated medications as potential treatments for Huntington's disease is being intensively pursued by pharmaceutical companies. Through the utilization of deuterium substitution's pharmacological advantages, these medications seek to enhance the effectiveness, safety, and acceptability of current therapies for HD symptoms. For instance, in August 2023, the FDA approved INGREZZA® (valbenazine) capsules for the treatment of adults with chorea linked to Huntington's disease (HD), according to a statement released by Neurocrine Biosciences, Inc. The only selective vesicular monoamine transporter 2 (VMAT2) inhibitor that provides a patient's doctor with an effective beginning dosage that may be modified depending on response and tolerability without requiring a complicated titration is INGREZZA. The only company that offers a straightforward dosage of one pill once a day is INGREZZA. The KINECT®-HD Phase 3 study and the continuing KINECT®-HD2 open-label extension trial, both carried out in cooperation with the Huntington Study Group (HSG), provided data that the FDA approved. KINECT-HD, a double-blind, randomized study that assessed the safety and effectiveness of INGREZZA, achieved its primary endpoint, which measured the change in chorea severity using the Total Maximal Chorea (TMC) score of the Unified Huntington's Disease Rating Scale (UHDRS) from baseline during the screening period to the maintenance period (average of Weeks 10 and 12). This change in TMC score was statistically significant when INGREZZA was used compared to placebo.
Deuterated Drugs Market Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Deuterated Drugs Market |
| Market Size in 2023 | USD 341.05 Million |
| Market Forecast in 2032 | USD 908.43 Million |
| Growth Rate | CAGR of 11.50% |
| Number of Pages | 222 |
| Key Companies Covered | Suzhou Zelgen Biopharmaceuticals, Teva, Vertex Pharmaceuticals, Otsuka Pharmaceutical (Avanir), Hinova Pharma, Concert Pharmaceuticals, Poxel SA (DeuteRx), Neuland Laboratories Ltd., and others. |
| Segments Covered | By Type, By Application, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Regional Analysis
North America is expected to dominate the market during the forecast period
North America is expected to dominate the market over the forecast period. Robust regulatory organizations like the U.S. Food and Drug Administration (FDA) and Health Canada are based in North America, especially the United States. They are essential in assessing the efficacy and safety of pharmaceutical goods, including deuterated medications. The development and commercialization route for deuterated pharmaceuticals in the area is shaped by the regulatory approval process.
Furthermore, there is a need for novel treatment options, such as deuterated pharmaceuticals, due to the high prevalence of various diseases and medical problems in North America. Deuterated pharmaceuticals have large commercial prospects in therapeutic areas like oncology, neurology, infectious illnesses, and metabolic disorders. These fields aim to address unmet medical needs and improve patient outcomes.
Deuterated Drugs Market: Competitive Analysis
The global deuterated drugs market is dominated by players like:
- Suzhou Zelgen Biopharmaceuticals
- Teva
- Vertex Pharmaceuticals
- Otsuka Pharmaceutical (Avanir)
- Hinova Pharma
- Concert Pharmaceuticals
- Poxel SA (DeuteRx)
- Neuland Laboratories Ltd.
The global deuterated drugs market is segmented as follows:
By Type
- Austedo
- Zepsun
- Others
By Application
- Cancer
- Huntington'S Disease (HD)
- Tardive Dyskinesia (TD)
- Others
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
A deuterated medication is a small molecular structure that has had one or more of its hydrogen atoms swapped out for the stable, heavier isotope deuterium. Deuteration has an impact on the drug's pharmacokinetic characteristics, including metabolism. Deuteration enhances the stability, safety, and tolerance of the medicine by decreasing its metabolism and strengthening its chemical bonds. Drugs that are deuterated are less poisonous, more effective, and have a longer half-life. Deuterated medications are used to treat tardive dyskinesia, cancer, and chorea (an involuntary movement disorder) linked to Huntington's disease. The first deuterated medicine molecule, deutetrabenazine, an analog of the previous medication tetrabenazine, has been approved for commercialization by the FDA. To treat chorea, one uses utetrabenazine.
The growing number of clinical trials, product approvals, prevalence of rare diseases, strong regulatory support and growing partnerships are expected to drive the deuterated drugs market during the forecast period.
According to the report, the global market size was worth around USD 341.05 million in 2023 and is predicted to grow to around USD 908.43 million by 2032.
The global deuterated drugs market is expected to grow at a CAGR of 11.50% during the forecast period.
The global deuterated drugs market growth is expected to be driven by North America. It is currently the world’s highest revenue-generating market due to the presence of regulatory agencies and the prevalence of rare diseases.
The global deuterated drugs market is dominated by players like Suzhou Zelgen Biopharmaceuticals, Teva, Vertex Pharmaceuticals, Otsuka Pharmaceutical (Avanir), Hinova Pharma, Concert Pharmaceuticals, Poxel SA (DeuteRx) and Neuland Laboratories Ltd. among others.
The deuterated drugs Market report covers the geographical market along with a comprehensive competitive landscape analysis. It also includes cash flow analysis, profit ratio analysis, market basket analysis, market attractiveness analysis, sentiment analysis, PESTLE analysis, trend analysis, SWOT analysis, trade area analysis, demand & supply analysis, Porter’s five forces analysis, and value chain analysis.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed