Electronic Data Capture Systems Market Size, Share, Trends, and Forecast 2032

Electronic Data Capture Systems Market By Delivery Mode (Web-hosted, Cloud-based, Licensed Enterprise), By Clinical Trial Phase (Phase I, Phase II, Phase III and Phase IV), By End-User (Contract Research Organizations (CROs), Pharmaceutical and Biotechnology Companies, Medical Device Companies and Hospitals), and By Region: Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2024-2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
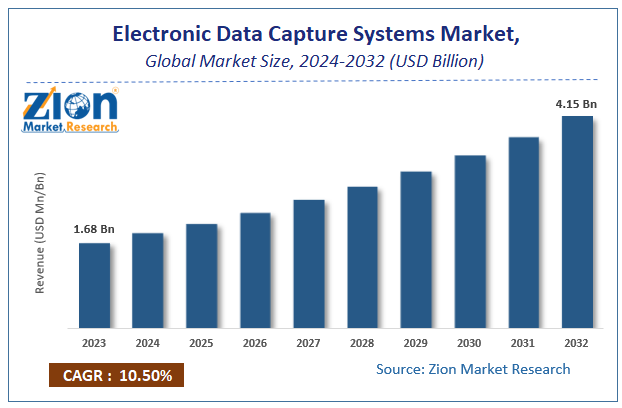
| USD 1.68 Billion | USD 4.15 Billion | 10.5% | 2023 |
Description
Electronic Data Capture Systems Market Industry Prospective
The global Electronic Data Capture Systems Market size was valued at USD 1.68 Billion in 2023 and is predicted to reach USD 4.15 Billion by the end of 2032. The market is expected to grow with a CAGR of 10.5% during the forecast period. The report analyzes the global Electronic Data Capture Systems Market's growth drivers, restraints, and impact on demand during the forecast period. It will also help navigate and explore the arising opportunities in the Electronic Data Capture Systems industry.
Global Electronic Data Capture System Market: Overview
Electronic data capture system is web-based software utilized to store all the data of a patient collected through tests and clinical trials. The data when reported on the paper is then captured and save on electronic data capture system. The surveys say that almost 80% clinical trials fail due to rising complexity in the data management which delays drug commercialization too because of unmet regulatory needs. As clinical research facilities and companies need to work more in less time, such electronic data capture systems are a good alternative as it saves time, gives accuracy, therefore, give fast results in less time. Therefore, the demand for electronic data capture systems is increasing in pharmaceutical companies and clinical research organizations.
Global Electronic Data Capture System Market: Growth Factors
Electronic data capture has become one of the preferred techniques for researchers and health professionals in clinical studies. Improvements in management of information & analysis need to compile technical, medical and scientific data clearly and precisely is contributing towards the requirement of electronic data capture which in turn is fostering the growth of the global electronic data capture system market. Clinical trials, medical devices, and electronic medical records all have significant growth potential thanks to eClinical systems, which provide a wide choice of workflow alternatives. These advantages include advice on how to interpret new findings in a medical context and the elimination of data discrepancies. Advanced statistical analysis and improved compilation of technical and scientific data required for regulatory agreement on clinical studies are also advantages of employing electronic data capture systems.
These are thought to improve the whole approval process and, as a result, increase the number of people who use EDC procedures in the future. All such factors are equally contributing to the growth of the global market. The most important factor for the growth of the global electronic data capture market is the rapid advancement of technology. Furthermore, to stay up with changes in the market, a big number of providers are always developing new EDC approaches. This is likely to boost the global electronic data capture system market growth during the forecast period. However, investing in electronic data capture system can be considered as a huge investment for small scale companies and start-ups, thus, the market may witness a bit decline in such cases. Also, as there are many alternative options, such as software-as-a-service which also work similarly and support the clinical research, which may create hindrance in the growth of the global electronic data capture system market.
In a primer need to streamline Covid-19 data collection and help in smooth process during the Covid-19, the companies tried making their reports with electronic data capture. The properties of electronic data capture system may help in Covid-19 research studies to understand the virus and help preventing future waves like it is doing now. The usage of EDC, therefore, grew during the pandemic thereby accelerating the growth of the global electronic data capture system market.
To know more about this report, request a sample copy.
Global Electronic Data Capture System Market: Segmentation
The global electronic data capture system market is segmented into components, delivery mode, development phase, end-user application, and region.
The global electronic data capture market, on the basis of components, is divided into software and services.
Based on delivery mode, the market is segmented into web-hosted, licensed enterprise, and cloud based.
The development phase segment is categorized into phase 1, phase 2, phase 3, and phase 4.
The end-user application in the market is segmented into hospitals, CROs, academic institutes, pharma & biotech organizations, and medical device manufacturers.
Electronic Data Capture Systems Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Electronic Data Capture Systems Market |
| Market Size in 2023 | USD 1.68 Billion |
| Market Forecast in 2032 | USD 4.15 Billion |
| Growth Rate | CAGR of 10.5% |
| Number of Pages | 211 |
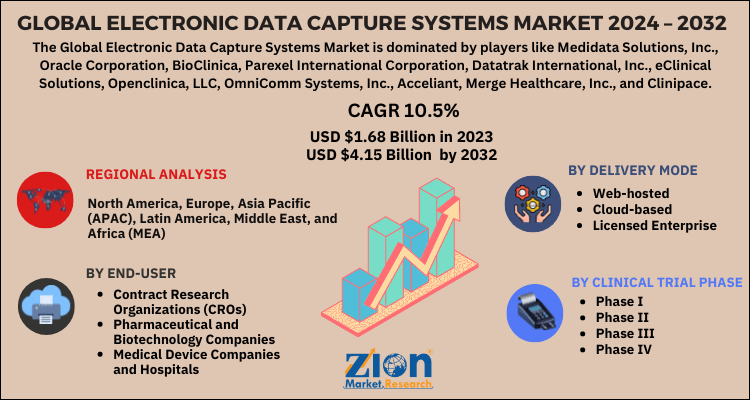
| Key Companies Covered | Medidata Solutions, Inc., Oracle Corporation, BioClinica, Parexel International Corporation, Datatrak International, Inc., eClinical Solutions, Openclinica, LLC, OmniComm Systems, Inc., Acceliant, Merge Healthcare, Inc., and Clinipace, among others |
| Segments Covered | By components, By delivery mode, By development phase, By end-user application and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Global Electronic Data Capture System Market: Regional Analysis
North America is expected to hold the largest share in global electronic data capture system market and remain dominating in the forecast period too. Stringent regulations by the government to handle clinical information confidentially and with safety, such data capture systems are growing in demand. Rising awareness regarding management of clinical information simply to meet regulatory requirements is another key growth prospect. Also, as large number of pharmaceutical companies are present in North America region, the adoption is of electronic data capture system is much better and therefore, the market is expected to have a lucrative growth. Asia Pacific is also anticipated to have a remarkable growth owing to presence of large number of clinical research organizations which provide clinical trial solutions and medical devices to various pharmaceutical companies. Furthermore, due to favorable government policies and technological advancements happening in Europe, global electronic data capture system market in the region is expected to have a significant growth.
Global Electronic Data Capture System Market: Competitive Players and Segments
The prominent manufacturers in the global electronic data capture system market include
- Medidata Solutions, Inc.
- Oracle Corporation
- BioClinica
- Parexel International Corporation
- Datatrak International, Inc.
- eClinical Solutions
- Openclinica, LLC
- OmniComm Systems, Inc.
- Acceliant
- Merge Healthcare, Inc.
- and Clinipace, among others.
By Delivery Mode
- Web-hosted
- Cloud-based
- Licensed Enterprise
By Clinical Trial Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By End-User
- Contract Research Organizations (CROs)
- Pharmaceutical and Biotechnology Companies
- Medical Device Companies and Hospitals
Global Electronic Data Capture System Market: Regional Segment Analysis
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
What Reports Provides
- Full in-depth analysis of the parent market
- Important changes in market dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional markets
- Testimonials to companies in order to fortify their foothold in the market.
Table Of Content
Choose License Type
List of Contents
Market Industry ProspectiveGlobal Electronic Data Capture System OverviewGlobal Electronic Data Capture System Growth FactorsGlobal Electronic Data Capture System SegmentationReport ScopeGlobal Electronic Data Capture System Regional AnalysisGlobal Electronic Data Capture System Competitive Players and SegmentsGlobal Electronic Data Capture System Regional Segment AnalysisWhat Reports ProvidesFrequentlyAsked Questions
Electronic data capture has become one of the preferred techniques for researchers and health professionals in clinical studies. Improvements in management of information and analysis, need to compile technical, medical and scientific data clearly and precisely is contributing towards the requirement of electronic data capture.
The prominent manufacturers in the sector include Medidata Solutions, Inc., Oracle Corporation, BioClinica, Parexel International Corporation, Datatrak International, Inc., eClinical Solutions, Openclinica, LLC, OmniComm Systems, Inc., Acceliant, Merge Healthcare, Inc., and Clinipace, among others.
Which region will make notable contributions towards overall electronic data capture systems market?
North America held the largest share in global electronic data capture system market during past years and is expected to remain dominating in the forecast period too. Stringent regulations by the government to handle clinical information confidentially and with safety, such data capture systems are growing in demand.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed