Fabry Disease Treatment Market Size, Share, Trends, Growth and Forecast 2030

Fabry Disease Treatment Market By Treatment (Substrate Reduction Therapy, Enzyme Replacement Therapy, Chaperone Treatment, and Others), By Caregiver Type (Hospitals, Specialty Clinics, and Others), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2023 - 2030
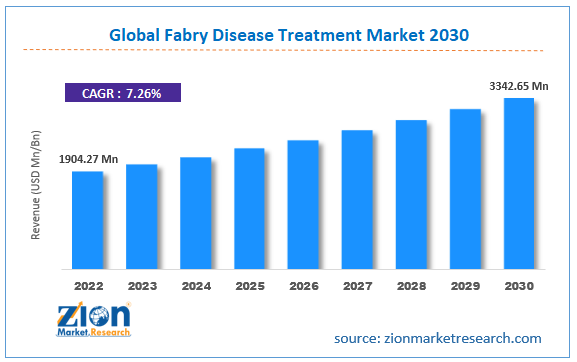
| Market Size in 2022 | Market Forecast in 2030 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 1904.27 Million | USD 3342.65 Million | 7.26% | 2022 |
Fabry Disease Treatment Industry Prospective:
The global Fabry disease treatment market size was worth around USD 1904.27 million in 2022 and is predicted to grow to around USD 3342.65 million by 2030 with a compound annual growth rate (CAGR) of roughly 7.26% between 2023 and 2030.
Fabry Disease Treatment Market: Overview
Fabry disease is a genetic disease passed on from the parents to children and is caused due to the buildup of globotriaosylceramide, a type of fat. The disease starts showing signs and symptoms at the beginning of childhood and some of the most commonly observed symptoms are frequent episodes of pain especially concentrated in body areas such as feet & hands, clusters of dark, small, red spots on the skin also known as angiokeratomas, cloudiness in front of the eyes, reduced ability to sweat or (hypohidrosis), and ringing in the ears. In addition to this, patients may also complain of hearing loss and problems with the gastrointestinal system. However, the signs are not limited to only these conditions as they may vary from one patient to another with specific groups showing other signs. If left untreated or undiagnosed, Fabry disease can also lead to life-threatening complications such as stroke, heart failure, and progressive kidney failure. In some patients, the condition may show milder symptoms that may appear in later stages of adulthood and may be concentrated only in areas including blood vessels in the brain, kidney, or heart.
Key Insights:
- As per the analysis shared by our research analyst, the global Fabry disease treatment market is estimated to grow annually at a CAGR of around 7.26% over the forecast period (2023-2030)
- In terms of revenue, the global Fabry disease treatment market size was valued at around USD 1904.27 million in 2022 and is projected to reach USD 3342.65 million, by 2030.
- The Fabry market is projected to grow at a significant rate due to the increasing prevalence of Fabry disease to create higher demand
- Based on caregiver type segmentation, hospitals were predicted to show maximum market share in the year 2022
- Based on treatment segmentation, enzyme replacement therapy was the leading segment in 2022
- On the basis of region, North America was the leading revenue generator in 2022
Fabry Disease Treatment Market: Growth Drivers
Increasing prevalence of Fabry disease to create higher demand for treatment
The global Fabry disease treatment market is projected to grow owing to the increasing prevalence of the condition. As per research analysis, the condition affects 1 in 1000 to 9000 people. The rising population rate increases the chances of the condition spreading to newborns since it is a hereditary disorder. Recent observations conclude that the chances of a person developing milder symptoms with age are more common than contracting the severe form. Moreover, people of all ethnicities and genders are vulnerable to Fabry disease leading to the condition affecting a broader group of patients. In addition to this, awareness around Fabry disease is growing rapidly as discussions in the scientific and medical community are on the rise. People have become more understanding of the associated symptoms leading to early diagnosis which plays a crucial role in driving the market revenue further.
Rising innovation in diagnostic technologies to help the industry expand further
Fabry disease is generally considered an under-diago condition and until a few years ago, appropriate diagnostic tools for determining Fabry disease in a patient with complete accuracy were difficult. However, with the ongoing technological advancements in the medical and healthcare industry, efficient and accurate diagnosis is possible. In August 2018, PerkinElmer received US Food and Drug Administration (FDA) approval for the commercial sale of NeoLSD MSMS Kit, a new tool with the capability to detect around 6 lysosomal storage disorders including Fabry disease in newborns. The test can be conducted using only blood samples.
Fabry Disease Treatment Market: Restraints
Lack of cure against Fabry disease to restrict market growth
The global Fabry disease treatment market growth may be restricted since there is no cure for the condition. The treatments available in the healthcare industry can also treat symptoms and alleviate associated pain. As of 2023, the only effective treatment available is enzyme replacement therapy (ERT). However, medical professionals may also recommend treatment such as adjunct therapies along with conventional medical treatment. It may include making changes in lifestyle, eating, and other habits. However, Fabry disease cannot be eliminated. Furthermore, since the treatment is long-lasting the cost is also relatively high thus limiting the number of patients that can undergo the treatment process.
Fabry Disease Treatment Market: Opportunities
Increasing approval of new treatment therapies for commercial application could create higher growth opportunities
The Fabry disease treatment industry players are expected to explore higher growth possibilities owing to the ongoing research in understanding the disease and the symptoms while also investing in developing novel therapies for treatment. Since the condition can impact a large segment of the growing population, more pharmaceutical players and healthcare agencies are working toward creating effective treatment plans along with improved diagnostic tools. In May 2023, the US FDA approved the use of pegunigalsidase alfa. It is an ERT to be used for treating confirmed cases of Fabry disease in adults.
Rising healthcare investment to provide scope for new growth avenues
The growing demand for adequate and quality healthcare has led to an increase in global healthcare expenditure with regional governments and international agencies working toward creating a healthcare ecosystem that is accessible to all. As per the World Health Organization, global health expenditure has reached a mark of USD 9 trillion.
Fabry Disease Treatment Market: Challenges
Lack of supporting healthcare infrastructure to challenge market growth
The Fabry disease treatment industry is likely to be challenged by the lack of supporting healthcare infrastructure especially in developing and underdeveloped countries. Nations with high population rates do not have effective healthcare programs since a large part of the population does not have access to primary medical care. Additionally, since the disease is not as widespread as other life-threatening conditions, a limited number of people are aware of the disease or available treatment. There is a significant gap in the supply and demand for skilled medical professionals who understand the condition thoroughly, further creating barriers against growth.
Fabry Disease Treatment Market: Segmentation
The global Fabry disease treatment market is segmented based on treatment, caregiver type, and region.
Based on treatment, the global Fabry disease treatment market segments are substrate reduction therapy, enzyme replacement therapy, chaperone treatment, and others. In 2022, the highest demand was observed for enzyme replacement therapy since the treatment is accepted across countries including Europe and North America. The rising number of ERT-oriented treatment programs has helped the segment grow further. For instance, using Fabrazyme during ERT helps in restoring alpha-galactosidase A levels thus allowing the body to break down lipids and provide relief from symptoms associated with Fabry disease. The average lifetime expectancy of male patients is around 58 years.
Based on caregiver type, the Fabry disease treatment industry divisions are hospitals, specialty clinics, and others. In 2022, the hospitals segment led with the highest growth rate. These units have higher patient footfall and are more equipped to provide treatments. Additionally, they also have access to advanced diagnostic tools and medical expertise. Growing healthcare investments may drive segmental growth. There are more than 15000 hospitals in the European Union.
Fabry Disease Treatment Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Fabry Disease Treatment Market |
| Market Size in 2022 | USD 1904.27 Million |
| Market Forecast in 2030 | USD 3342.65 Million |
| Growth Rate | CAGR of 7.26% |
| Number of Pages | 230 |
| Key Companies Covered | JCR Pharmaceuticals, Sanofi Genzyme, Green Cross Corporation, Chiesi Group, Regenxbio Inc., Protalix BioTherapeutics, Idorsia Pharmaceuticals, Amicus Therapeutics, and others. |
| Segments Covered | By Treatment, By Treatment, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2022 |
| Historical Year | 2017 to 2021 |
| Forecast Year | 2023 - 2030 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Fabry Disease Treatment Market: Regional Analysis
North America to witness the highest growth rate in the near future
The global Fabry disease treatment market is most likely to be dominated by North America due to the presence of excellent healthcare infrastructure in the US. The regional pharmaceutical industry is innovation-driven focusing on the development of novel medical treatments for all types of medical conditions. Additionally, the increasing rate of FDA approvals for Fabry disease treatments has helped the region maintain its dominance. In September 2022, the agency granted the Orphan Drug Designation (ODD) to AL01211 produced by AceLink Therapeutics for treating Fabry disease. It is a glucosylceramide synthase (GCS) inhibitor and has shown high potency. The treatment was claimed as a much-needed orally consumed medicine as compared to other available treatments. Europe, on the other hand, is projected to grow at a significant rate. In May 2023, Europe allowed Chiesi Farmaceutici and Protalix BioTherapeutics to market their product PRX-102 (pegunigalsidase alfa) in the European territory as a treatment for Fabry disease.
Fabry Disease Treatment Market: Competitive Analysis
The global Fabry disease treatment market is led by players like:
- JCR Pharmaceuticals
- Sanofi Genzyme
- Green Cross Corporation
- Chiesi Group
- Regenxbio Inc.
- Protalix BioTherapeutics
- Idorsia Pharmaceuticals
- Amicus Therapeutics
The global Fabry disease treatment market is segmented as follows:
By Treatment
- Substrate Reduction Therapy
- Enzyme Replacement Therapy
- Chaperone Treatment
- Others
By Caregiver Type
- Hospitals
- Specialty Clinics
- Others
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Fabry disease is a genetic disease passed on from the parents to children and is caused due to the buildup of globotriaosylceramide, a type of fat.
The global Fabry disease treatment market is projected to grow owing to the increasing prevalence of the condition.
According to study, the global Fabry disease treatment market size was worth around USD 1904.27 million in 2022 and is predicted to grow to around USD 3342.65 million by 2030.
The CAGR value of Fabry disease treatment market is expected to be around 7.26% during 2023-2030.
The global Fabry disease treatment market is most likely to be dominated by North America due to the presence of excellent healthcare infrastructure in the US.
The global Fabry disease treatment market is led by players like JCR Pharmaceuticals, Sanofi Genzyme, Green Cross Corporation, Chiesi Group, Regenxbio Inc., Protalix BioTherapeutics, Idorsia Pharmaceuticals, Amicus Therapeutics, and many more
The global Fabry disease treatment market explores crucial aspects of the Fabry disease treatment market including a detailed discussion of existing growth factors and restraints while also browsing future growth opportunities and challenges that impact the Fabry disease treatment market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed