Fecal Transplant Therapy Market Size, Share, Industry Analysis, Growth, Forecasts, 2032

Fecal Transplant Therapy Market By Disease (Clostridium Difficile Infection, Autism, Obesity, Parkinson's Disease (PD), Diabetes Mellitus, and Others), By Procedure (Fecal Enema, Fecal Bacteriotherapy, Stool Transplant, Human Probiotic Infusion, and Fecal Transfusion), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2024 - 2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 2,058.20 Million | USD 3,248.20 Million | 5.20% | 2023 |
Fecal Transplant Therapy Industry Prospective:
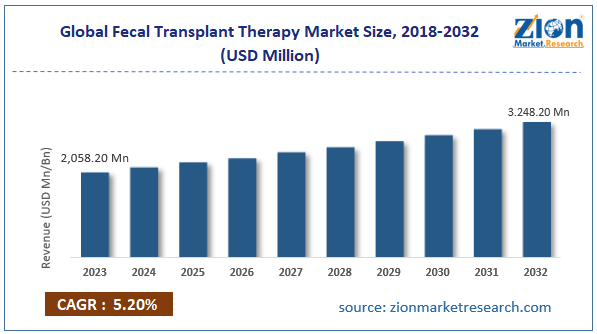
The global fecal transplant therapy market size was worth around USD 2,058.20 million in 2023 and is predicted to grow to around USD 3,248.20 million by 2032 with a compound annual growth rate (CAGR) of roughly 5.20% between 2024 and 2032.
Fecal Transplant Therapy Market: Overview
Fecal transplant therapy is a medical procedure. It deals with the collection of stool from healthy donors and carefully introducing it into the gastrointestinal tract of a patient. The procedure is mostly used to treat patients with Clostridium difficile also known as C. diff infection. Fecal transplant therapy helps in introducing healthy bacteria into the patient's intestines further helping with the proper functioning of the body organ. Fecal transplant processes can be carried out in adults and children. C. diff infection is caused when certain antibiotics impact the quantity of good bacteria found in the intestines. This leads to the bad bacteria also known as Clostridium difficile outnumbering the good bacteria quantity. Problems associated with C. diff infection include cramping, diarrhea, and fever.
One of the leading causes of Clostridium difficile infection is being treated for infection and consuming specific medicines for the treatment. It is most commonly observed in people over the age of 65 years or patients with chronic illnesses. In some cases, C. diff, if left untreated, can also turn fatal. In addition to fecal transplant acting as a prominent therapy for C. diff infection, it is also being reviewed to understand its efficiency in treating other conditions such as obesity and autism. During the forecast period, the demand in the fecal transplant therapy market is expected to grow at a steady rate.
Key Insights:
- As per the analysis shared by our research analyst, the global fecal transplant therapy market is estimated to grow annually at a CAGR of around 5.20% over the forecast period (2024-2032)
- In terms of revenue, the global fecal transplant therapy market size was valued at around USD 2,058.20 million in 2023 and is projected to reach USD 3,248.20 million, by 2032.
- The market is projected to grow at a significant rate due to the growing cases of C. diff infections
- Based on disease segmentation, clostridium difficile infection was predicted to show maximum market share in the year 2023
- Based on procedure segmentation, fecal bacteriotherapy was the leading procedure in 2023
- On the basis of region, Europe was the leading revenue generator in 2023
 Request Free Sample
Request Free Sample
Fecal Transplant Therapy Market: Growth Drivers
Growing cases of C. diff infections may fuel the market expansion rate
The global fecal transplant therapy market is expected to grow owing to the rising cases of C. diff infections across the globe. The condition can impact people of all genders and age groups. It is mostly common in patients over the age of 65 years. Other risk factors often associated with C. diff infection include recent visits to a nursing hospital or an extremely weak immune system. Several medical conditions are known to greatly reduce the strength of a patient's immune system. Medical issues such as cancer, Human immunodeficiency virus infection (HIV), and acquired immune deficiency syndrome (AIDS) are examples of issues known to directly affect a patient’s immunity level.
Additionally, one in every 6 patients who have been previously affected by C. diff is at the risk of being re-infected within a span of 2 or 8 weeks of previous treatment. As awareness about C. diff is on the rise, the demand for efficient treatment processes is simultaneously growing. Pfizer, a leading giant in the pharmaceutical sector, initiated a new panel called 'Clostridioides difficile: A public health threat in plain sight' in a recent event. This panel was attended by a host of patient organizations and medical professionals. The discussions held during the panel session were related to understanding more about C. diff and creating awareness about available treatment programs along with the scope of development of new processes in the coming years. Several studies have been published online and across research centers detailing the focus on the efficiency of fecal transplant therapy in treating the condition.
Increasing launch of new processes, medicines, and tools used during fecal transplant therapy may generate higher revenue
The efficiency of fecal transplant therapy in treating certain medical conditions has propelled more players to enter the global fecal transplant therapy market. The rise in the launch of new solutions, medicines, and tools that improve the success rate of fecal transfer processes will act as a crucial driver for the industry's growth trajectory.
In April 2023, the U.S. Food and Drug Administration (FDA) approved the use of the first pill to be used during fecal transplant therapy. The drug is called Vowst and can prevent the recurrence of C. diff infection.
Fecal Transplant Therapy Market: Restraints
Limitations of conventional fecal transplant may restrict the market expansion rate
The global fecal transplant therapy industry is restricted due to several limitations associated with conventional therapy processes dealing with fecal transplants. The most common disadvantages of the process include stool toxicity, pathogen transfer, and difficulty in reproduction. Additionally, although extremely rare, certain adverse reactions to fecal transplant therapy have also been reported worldwide.
Some of these reports deal with reactions such as pneumonia, ileus, and death. It is important to note that fecal transplant therapy is considered safe when conducted under the right guidance and by certified medical professionals equipped with the right tools.
Fecal Transplant Therapy Market: Opportunities
Rising investments in the healthcare sector will generate high-growth opportunities
The global fecal transplant therapy market will witness higher growth avenues as a result of increased expenditure toward improvements in the global healthcare sector along with programs to make quality healthcare accessible to all. In February 2024, the Sanofi Global Health Unit (GHU) announced its plans to invest in Africa’s healthcare sector by providing necessary financial aid to start-ups in the medical sector.
In addition to this, there is a growing rate of investment observed toward healthcare technology with a prime focus on integrating modern tools such as Artificial Intelligence, telemedicine, and novel diagnostic methods to detect and treat medical conditions. In December 2023, the World Health Organization (WHO) published its annual global health expenditure report and emphasized the need to invest more in universal health coverage.
Surging research & development to learn extended applications of fecal transplant therapy may be beneficial
The global fecal transplant therapy industry has high growth potential as more research & development are underway to learn about the extended applications of the procedure to treat other serious conditions such as obesity and autism. However, currently, there is no scientific proof that can confirm the efficiency of the treatment process for these conditions. Extensive research indicates that fecal transplant therapy is highly beneficial in treating recurrent C. diff infections.
A study published in 2021 by the National Cancer Institute indicated that fecal microbiota transplants can help patients suffering from advanced melanoma respond to immunotherapy.
Fecal Transplant Therapy Market: Challenges
Relatively new acceptance rate of the process in the medical community is a major challenge
The global fecal transplant industry is relatively new in the healthcare industry. Although the procedure has been documented for centuries, modern medicine is new to the treatment process resulting in a limited number of facilities and skilled professionals currently undertaking the process. Additionally, the absence of wide-scale medical infrastructure and the rising cost of medical procedures are the existing challenges in the industry.
Fecal Transplant Therapy Market: Segmentation
The global fecal transplant therapy market is segmented based on disease, procedure, and region.
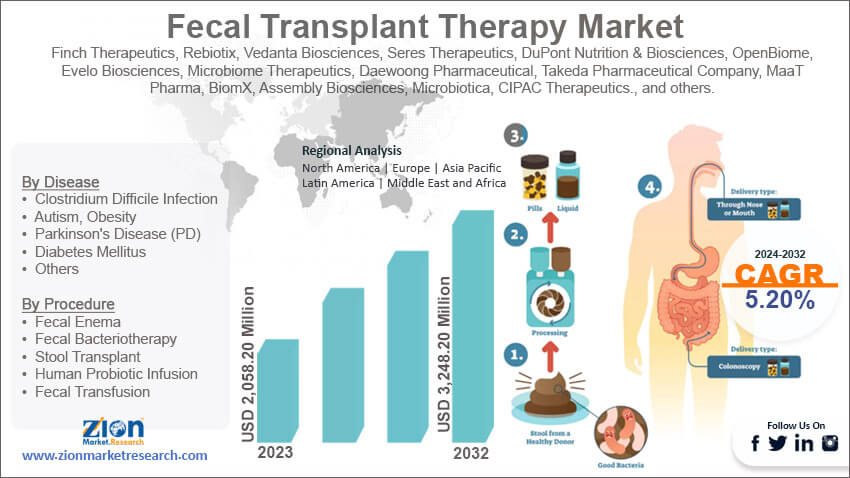
Based on disease, the global market segments are Clostridium difficile infection, autism, obesity, Parkinson’s disease (PD), diabetes mellitus, and others. The treatment process is mainly recommended to be used for treating Clostridium difficile infection. Several research studies conducted globally have proven the efficiency of fecal transplant therapy in treating C. diff infections. Research indicates that the success rate of fecal transplant in C. diff treatments is around 80% to 95%. Scholars are conducting more research to understand if the process can be used for treating other diseases.
Based on procedure, the global market is divided into fecal enema, fecal bacteriotherapy, stool transplant, human probiotic infusion, and fecal transfusion. In 2023, the highest growth was witnessed in the fecal bacteriotherapy segment. It dominated nearly 31.25% of the segmental share. The primary growth drivers are the increase in patients with target medical conditions and growing awareness about the effectiveness of the procedure in treating the condition.
Fecal Transplant Therapy Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Fecal Transplant Therapy Market |
| Market Size in 2023 | USD 2,058.20 Million |
| Market Forecast in 2032 | USD 3,248.20 Million |
| Growth Rate | CAGR of 5.20% |
| Number of Pages | 217 |
| Key Companies Covered | Finch Therapeutics, Rebiotix, Vedanta Biosciences, Seres Therapeutics, DuPont Nutrition & Biosciences, OpenBiome, Evelo Biosciences, Microbiome Therapeutics, Daewoong Pharmaceutical, Takeda Pharmaceutical Company, MaaT Pharma, BiomX, Assembly Biosciences, Microbiotica, CIPAC Therapeutics., and others. |
| Segments Covered | By Disease, By Procedure, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Fecal Transplant Therapy Market: Regional Analysis
Europe will act as an essential growth driver during the projection period
The global fecal transplant therapy market will be dominated by Europe during the projection period. The region has a higher awareness rate about C. diff infections. Several studies have been conducted throughout the region to obtain better insights into the condition, its causes, and treatment plans. As per the European Center for Disease Prevention and Control, the countries with the highest C. diff infection prevalence rate are Hungary, Poland, and Slovenia. For instance, Hungary’s prevalence rate is around 67.6%. In addition to this, the region also has a robust healthcare infrastructure with each citizen having access to preliminary medical care. In 2021, Spain spent nearly 54 billion euros on public healthcare. North America may witness a higher growth rate as compared to previous years. The United States recently approved the use of fecal transplant therapy for treating C. diff infection among patients.
As of February 2024, the American Gastroenterological Association has recommended fecal microbiota transplant (FMT) in the majority of cases of C. diff. Asia-Pacific is likely to emerge as the fastest-growing region with an increase in regional healthcare expenditure. Additionally, the growing medical tourism rate will further propel the regional market growth rate.
Fecal Transplant Therapy Market: Competitive Analysis
The global fecal transplant therapy market is led by players like:
- Finch Therapeutics
- Rebiotix
- Vedanta Biosciences
- Seres Therapeutics
- DuPont Nutrition & Biosciences
- OpenBiome
- Evelo Biosciences
- Microbiome Therapeutics
- Daewoong Pharmaceutical
- Takeda Pharmaceutical Company
- MaaT Pharma
- BiomX
- Assembly Biosciences
- Microbiotica
- CIPAC Therapeutics.
The global fecal transplant therapy market is segmented as follows:
By Disease
- Clostridium Difficile Infection
- Autism, Obesity
- Parkinson's Disease (PD)
- Diabetes Mellitus
- Others
By Procedure
- Fecal Enema
- Fecal Bacteriotherapy
- Stool Transplant
- Human Probiotic Infusion
- Fecal Transfusion
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Fecal transplant therapy is a medical procedure. It deals with the collection of stool from healthy donors and carefully introducing it into the gastrointestinal tract of a patient.
The global fecal transplant therapy market is expected to grow owing to the rising cases of C. diff infections across the globe.
According to study, the global fecal transplant therapy market size was worth around USD 2,058.20 million in 2023 and is predicted to grow to around USD 3,248.20 million by 2032.
The CAGR value of fecal transplant therapy market is expected to be around 5.20% during 2024-2032.
The global fecal transplant therapy market will be dominated by Europe during the projection period.
The global fecal transplant therapy market is led by players like Finch Therapeutics, Rebiotix, Vedanta Biosciences, Seres Therapeutics, DuPont Nutrition & Biosciences, OpenBiome, Evelo Biosciences, Microbiome Therapeutics, Daewoong Pharmaceutical, Takeda Pharmaceutical Company, MaaT Pharma, BiomX, Assembly Biosciences, Microbiotica, and CIPAC Therapeutics.
The report explores crucial aspects of the fecal transplant therapy market including detailed discussion of existing growth factors and restraints while also browsing future growth opportunities and challenges that impact the market.
Choose License Type
List of Contents
Fecal Transplant TherapyIndustry Prospective:OverviewKey Insights:Growth DriversIncreasing launch of new processes, medicines, and tools used during fecal transplant therapy may generate higher revenueRestraintsOpportunitiesChallengesSegmentationReport ScopeRegional AnalysisCompetitive AnalysisThe global fecal transplant therapy market is segmented as follows:By RegionHappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed