Healthcare Regulatory Affairs Outsourcing Market Size, Share, Growth Report 2032

Healthcare Regulatory Affairs Outsourcing Market- By Services (Regulatory Writing & Publishing, Clinical Trial Applications & Services Registrations, Regulatory Consulting & Legal Representation, and Regulatory Submissions), By End-User (Mid-Size Pharmaceutical Companies, Large Pharmaceutical Companies, Biotechnology Companies, Medical Device Companies, and Food & Beverage Companies), And By Region- Global Industry Perspective, Comprehensive Analysis, and Forecast, 2024 - 2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 7.55 Billion | USD 18.86 Billion | 10.7% | 2023 |
Healthcare Regulatory Affairs Outsourcing Market Insights
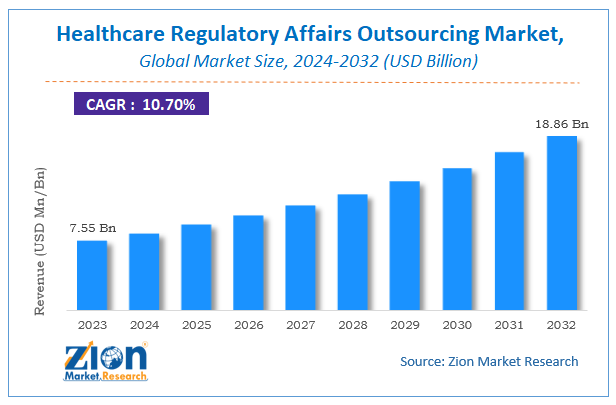
Zion Market Research has published a report on the global Healthcare Regulatory Affairs Outsourcing Market, estimating its value at USD 7.55 Billion in 2023, with projections indicating that it will reach USD 18.86 Billion by 2032. The market is expected to expand at a compound annual growth rate (CAGR) of 10.7% over the forecast period 2024-2032. The report explores the factors fueling market growth, the hitches that could hamper this expansion, and the opportunities that may arise in the Healthcare Regulatory Affairs Outsourcing Market industry. Additionally, it offers a detailed analysis of how these elements will affect market demand dynamics and market performance throughout the forecast period.
Healthcare Regulatory Affairs Outsourcing Market: Overview
Emerging biotech & pharmacy markets across globe has resulted into necessity for healthcare regulatory affairs outsourcing activities. Moreover, pharmacy firms are working with contract research organizations for new drug development. Surge in incidences of chronic disorders will generate new growth avenues for healthcare regulatory affairs outsourcing industry in coming years. Apparently, period of drug approval is very expensive and time-consuming and requires tedious process of documentation. However, to prevent these issues firms are entering into alliances with firms in business of healthcare regulatory affairs outsourcing for quick documentation & drug approval. This has translated into humungous demand for healthcare regulatory affairs outsourcing activities.
With the rising demand for various services, the market for healthcare regulatory affairs outsourcing is at upsurge. It includes writing and publishing of medical regulatory documentation which is offered by experienced quality control publishers and auditors and medical writers, who play an important role in the development of high-quality documents for clinical research projects.
Regulatory submissions refer to submission of any information or documentation related to healthcare products to a regulatory body in order to get it reviewed. There are also other outsourcing services like regulatory consulting, legal representations, and clinical trial applications. All these services are in high demand from all the end-user companies ranging from pharma to food and beverages.
Many countries are increasing their investments in the research and development activities which have triggered the development and launch of new products in the market. With the rising guidelines by the regulatory authorities all over the world, there has been a growing alliance between the drug developers, CRO’s and CMO’s to mitigate risks. Since CMO’s and CRO’s help the companies in these risks, companies are able to focus on their core businesses. Therefore, patent expirations, along with increasing R&D activities have been identified as the major factors fueling the growth of the healthcare regulatory affairs outsourcing market.
The rise in demand for services offered by healthcare regulatory affairs outsourcing market has also resulted in increasing documentation during device and drug manufacturing. Thus the small and mid-sized pharmaceutical companies are facing problems of extensive documentation. Due to the stringent policies, the regulatory authorities demand skilled and trained regulatory professionals who are capable of handling registration, evaluation, and compilation of data efficiently. Consequently, the biotechnology and pharmaceutical companies are seeking help from the regulatory service providers, thus boosting the market growth for healthcare regulatory affairs outsourcing.
Healthcare Regulatory Affairs Outsourcing Market: Growth Drivers
Constant surge in compliance measures, strict legislations governing drug development & approvals, and high proportion of medicine withdrawals has compelled biotech, pharm, and life science firms to outsource healthcare regulatory affairs activities with a view to reduce operating costs. This, in turn, will enlarge scope of growth of healthcare regulatory affairs outsourcing industry over forecasting period. In addition to this, regulatory support is necessary for large number of processes in clinical studies including manufacturing & controls methods, healthcare report writing, data management processes, regulatory chemistry procedures, and labeling & liaison methods. This will proliferate size of healthcare regulatory affairs outsourcing industry over assessment period. Moreover, outsourcing of non-core activities will help firms concentrate on various research & development activities.
In addition to this, outsourcing of healthcare regulatory affairs is predicted to assist firms in focusing on core competencies, minimizing service delivery period, and enhance competitive edge for firms. This will create new growth avenues for healthcare regulatory affairs outsourcing market over forecasting timespan. Nevertheless, huge risks related to data security issues in firms can pose a threat to expansion of healthcare regulatory affairs outsourcing industry in foreseeable future.
Healthcare Regulatory Affairs Outsourcing Market: Segmentation
The healthcare regulatory affairs outsourcing market is segmented based on services, end-user and by region. All the segments have been analyzed based on present and future trends and the market is estimated from 2024 to 2032.
Based on services, the market is segmented into regulatory writing and publishing, regulatory submissions, legal representation, and regulatory consulting, clinical trial applications and services registrations, and other regulatory affairs.
The end-user segment is divided into biotechnology companies, mid-size pharmaceutical companies, medical device companies, large pharmaceutical companies, and food and beverage companies.
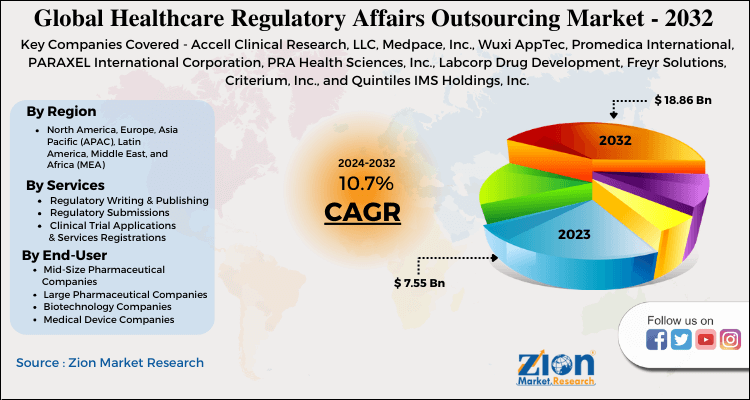
The regional segment, North America and Europe hold the largest share in the healthcare regulatory affairs outsourcing market. This growth is mainly attributed to the huge number of R&D activities in the field of drug discovery carried out in these two regions. These regions also have a large presence of multinational biotech and pharma companies which carry out a large number of clinical trials and also have well developed and advanced healthcare infrastructure. Due to high healthcare investment and rising government funding Europe also holds a maximum share of the market followed by North America. Asia Pacific region is also witnessing significant growth rate owing to the unmet health care needs and raised government funding towards research activities. Also, many biotech and pharma companies have started outsourcing their drug development services to various developing countries like China, Malaysia, India, and Singapore which is further boosting the market growth for healthcare regulatory affairs outsourcing market.
Healthcare Regulatory Affairs Outsourcing Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Healthcare Regulatory Affairs Outsourcing Market |
| Market Size in 2023 | USD 7.55 Billion |
| Market Forecast in 2032 | USD 18.86 Billion |
| Growth Rate | CAGR of 10.7% |
| Number of Pages | 193 |
| Key Companies Covered | Accell Clinical Research, LLC, Medpace, Inc., Wuxi AppTec, Promedica International, PARAXEL International Corporation, PRA Health Sciences, Inc., Labcorp Drug Development, Freyr Solutions, Criterium, Inc., and Quintiles IMS Holdings, Inc. |
| Segments Covered | By Services, By End-User and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Healthcare Regulatory Affairs Outsourcing Market: Regional Landscape
North America To Account Majorly Towards Overall Market Size By 2032
Growth of healthcare regulatory affairs outsourcing market in North America over assessment period can be due to surge in number of copyright expiries and focus on new product launches in countries such as the U.S. and Canada. Apart from this, need for quick drug approvals to gain competitive advantage over business rivals and acquire major industry share has resulted in healthcare firms outsource healthcare regulatory affairs activities in countries such as the U.S. This has translated into humungous growth of regional market.
Competitive Landscape
Some of the leading players in healthcare regulatory affairs outsourcing market include Medpace, Inc., Promedica International, PARAXEL International Corporation, Covance, Inc., Freyr Solutions, Criterium, Inc., Accell Clinical Research, PRA Health Sciences, Inc., Wuxi AppTec and Quintiles IMS Holdings, Inc. There has been a trend of a strategic partnership between services providers for increasing service portfolio to expand regional presence. For instance, Shin Nippon Biomedical Laboratories Ltd. (SNBL), leading provider of development services to biopharmaceuticals, formed a joint venture with PPD to provide clinical development services in Japan which includes function-based services such as pharmacovigilance.
Key players profiled in study and influencing market growth are:
- Accell Clinical Research, LLC
- Medpace, Inc.
- Wuxi AppTec
- Promedica International
- PARAXEL International Corporation
- PRA Health Sciences, Inc.
- Labcorp Drug Development
- Freyr Solutions
- Criterium, Inc.
- Quintiles IMS Holdings, Inc.
The global Healthcare Regulatory Affairs Outsourcing Market is segmented as follows:
By Services
- Regulatory Writing & Publishing
- Regulatory Submissions
- Clinical Trial Applications & Services Registrations
- Regulatory Consulting & Legal Representation
By End-User
- Mid-Size Pharmaceutical Companies
- Large Pharmaceutical Companies
- Biotechnology Companies
- Medical Device Companies
- Food & Beverage Companies
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Constant surge in compliance measures, strict legislations governing drug development & approvals, and high proportion of medicine withdrawals has compelled biotech, pharm, and life science firms to outsource healthcare regulatory affairs activities with a view to reduce operating costs. This, in turn, will enlarge scope of growth of healthcare regulatory affairs outsourcing industry over forecasting period. In addition to this, regulatory support is necessary for large number of processes in clinical studies including manufacturing & controls methods, healthcare report writing, data management processes, regulatory chemistry procedures, and labeling & liaison methods. This will proliferate size of healthcare regulatory affairs outsourcing industry over assessment period. Moreover, outsourcing of non-core activities will help firms concentrate on various research & development activities.
In addition to this, outsourcing of healthcare regulatory affairs is predicted to assist firms in focusing on core competencies, minimizing service delivery period, and enhance competitive edge for firms. This will create new growth avenues for healthcare regulatory affairs outsourcing market over forecasting timespan.
According to Zion market research report, the global Healthcare Regulatory Affairs Outsourcing Market accrued earnings worth approximately US$ 7.55 Billion in 2023, to US$ 18.86 Billion by 2032 at a CAGR of about 10.7% from 2024 to 2032.
North America will contribute lucratively towards the global market earnings over the projected timeline. The regional market surge is subject to surge in number of copyright expiries and focus on new product launches in countries such as the U.S. and Canada. Apart from this, need for quick drug approvals to gain competitive advantage over business rivals and acquire major industry share has resulted in healthcare firms outsource healthcare regulatory affairs activities in countries such as the U.S. This has translated into humungous growth of regional market.
The key market participants include Accell Clinical Research, LLC, Medpace, Inc., Wuxi AppTec, Promedica International, PARAXEL International Corporation, PRA Health Sciences, Inc., Labcorp Drug Development, Freyr Solutions, Criterium, Inc., and Quintiles IMS Holdings, Inc.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed