Heart Pump Device Market Size, Share, Growth & Trends 2028

Heart Pump Device Market by Product (Ventricular Assist Devices, Intra-aortic Balloon Pumps, Total Artificial Hearts), by Type (Implanted Heart Pump Device, Extracorporeal Heart Pump Device), by Therapy (Bridge-to-Transplant (BTT), Bridge-to-Candidacy (BTC), Destination Therapy (DT), Other Therapies), and By Region: Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data and Forecasts 2022 - 2028
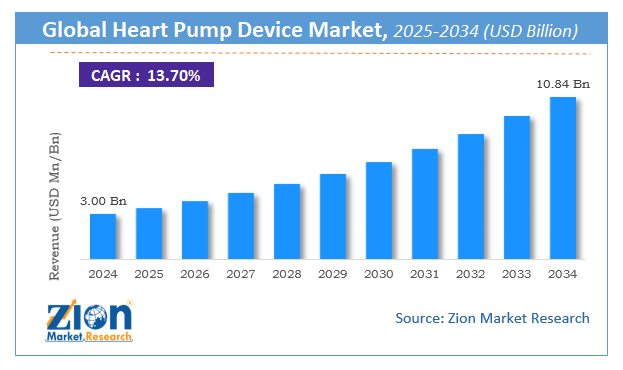
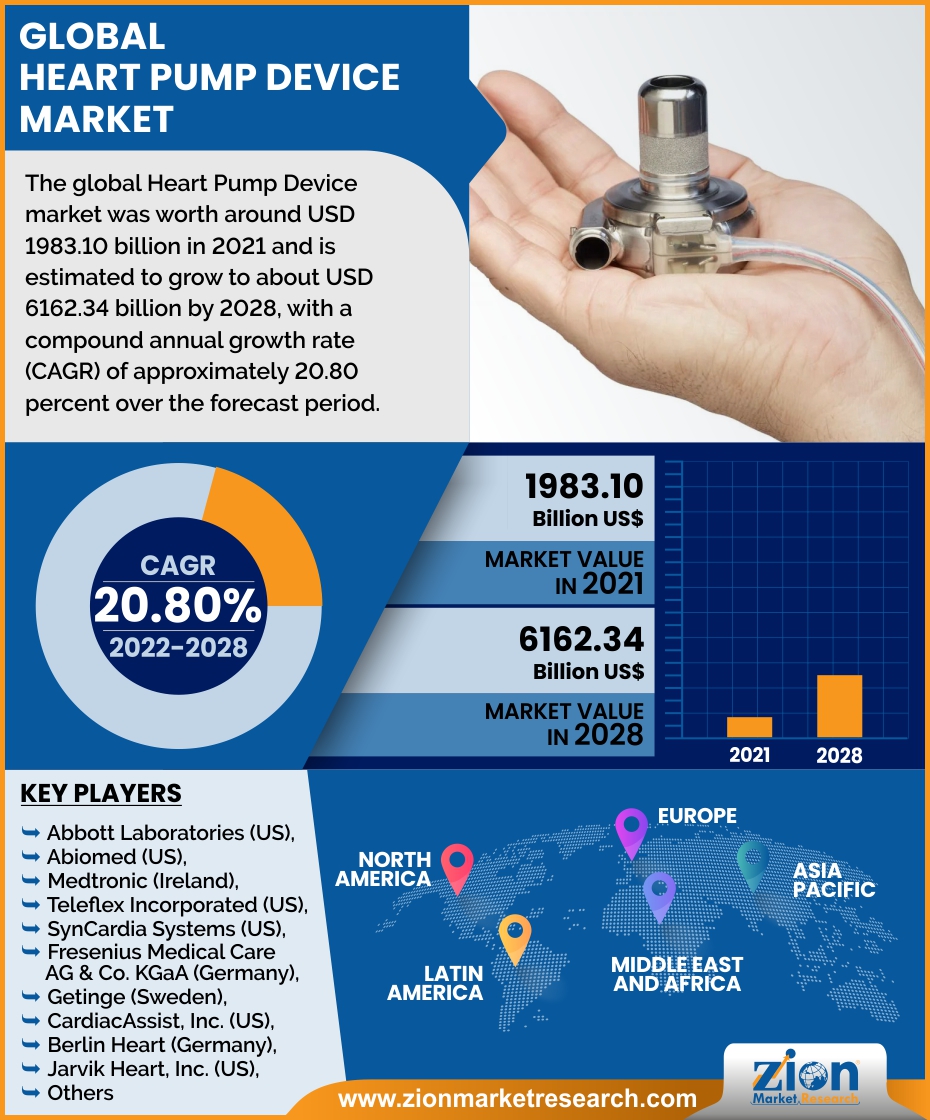
The global Heart Pump Device market valued USD 1983.10 billion in 2021 and estimated to USD 6162.34 billion by 2028, with a (CAGR) of 20.80 %.
Heart Pump Device Market Size
The global Heart Pump Device market was worth around USD 1983.10 billion in 2021 and is estimated to grow to about USD 6162.34 billion by 2028, with a compound annual growth rate (CAGR) of approximately 20.80 percent over the forecast period. The report analyzes the Heart Pump Device market’s drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the Heart Pump Device market.
Heart Pump Device Market: Overview
Heart failure occurs when the heart is unable to pump enough blood to meet the body's requirements. This syndrome could be caused by coronary artery disease, such as a recent myocardial infarction (heart attack), high blood pressure, atrial fibrillation, valvular heart disease, and other factors. Many different types of heart pump devices are available on the market to assist cardiac circulation, depending on the cardiovascular disease. They are surgically implanted and used to provide temporary or long-term support for heart function in persons who have a weak heart or erratic blood flow. Several people may benefit from heart pump devices as a treatment alternative for mechanical circulatory assistance.
The rise in the prevalence of cardiovascular illnesses, the increase in the elderly population, and the increase in new product advancements by industry participants are all driving the global heart pump device market forward. Due to a scarcity of heart donors, heart pump devices serve as a viable alternative, fueling the global market's expansion. During the projection period, however, constraints such as the high cost of heart pump devices and the poor reimbursement situation in developing countries are projected to limit market growth. Furthermore, the market's growth is hampered by turbulence and high shear stresses associated with mechanical heart valves (MHVs), as well as the need for patients to take constant anti-coagulation therapy.
COVID-19 Impact:
Following the global epidemic of COVID-19, there have been considerable changes in the healthcare system. Several countries have agreed to postpone elective surgery in the cardiac care industry to reduce the risk of infection. Elective cardiac procedures have been postponed in the UK because to the impact of COVID19 on healthcare systems. HF admissions were dramatically reduced during the lockdown, according to a study done across eight Italian hospitals from February 21 to March 31, 2020. HF patients may have died at home without seeking medical attention during the COVID19 lockdown in some situations.With fewer HF hospitalizations, the market for heart pump devices is expected to fall significantly in 2020. Similarly, the breakout of COVID-19 has resulted in a drop in revenue for market players.
According to Abiomed's 10-k form, revenues from Impella pump sales were impacted in the fourth quarter of FY 2020 due to low patient utilisation as a result of fewer patient visits. COVID-19 reduced Impella's revenue by around USD 17 million, largely in the United States and Europe. The FDA has granted emergency use authorisation (EUA) to firms that manufacture ventricular assist devices to treat COVID patients with right heart failure or decompensation, including pulmonary embolism, as a result of the COVID epidemic (PE).Abiomed got an EUA from the FDA in August 2020 for the left-sided Impella heart pumps, which offer left ventricular unloading and support COVID-19 patients on ECMO. Impella RP also got an EUA from the FDA, allowing it to be used in patients with COVID-19-related right heart failure or decompensation. These advancements aided the company's significant expansion.
Heart Pump Device Market: Growth Drivers
Long waiting periods for heart transplant procedures to drive global market growth
Due to the rising incidence of CVDs and heart failure in recent years, the demand for heart transplantation has increased dramatically. However, a global shortage of suitable hearts for transplantation has developed from the inability to meet current demand. As of March 2018, there were around 3,980 candidates on the heart transplant waiting list, according to the national database kept by the Health Resources and Services Administration (US). Significant disparities in the number of donors and patients on waiting lists have been seen even at the country level. In Australia, for example, around 1,650 people were on the waiting list for a heart transplant in 2020, but only 463 donors were available (Source: Transplant Australia Ltd).According to the Transplant Activity Report 2018/19, the number of patients on heart transplant waiting lists had climbed by 134% since 2010. In this situation, not only has the number of patients on the transplant waiting list increased, but so has the number of patients who have died while on the waiting list.
As a bridge-to-transplantation (BTT) or destination therapy for advanced HF, LVADs have become crucial tools (DT). The expanding number of end-stage HF patients, combined with technological advancements in mechanical circulatory support (MCS), has raised demand for MCS devices used in these patients. Between 2009 and 2016, MCS, LVAD, RVAD, TAH, and ECMO were used to bridge 43 percent of beneficiaries. These devices give patients with the required support while they wait for a heart transplant or as a long-term option for those who are not candidates for a heart transplant. As a result of the considerable gap between demand and supply of donor hearts, the heart pump devices market is likely to rise in the future years.
Heart Pump Device Market: Restraints
High cost of heart pump device equipment in Heart Pump Devices to hamper the market growth
For bridge-to-heart transplantation, ventricular support devices, notably left ventricular assist devices (LVADs), are commonly employed. Although LVADs are considered life-saving devices, they do come with a number of drawbacks. By six months after implantation, up to 60% of patients have experienced LVAD-related problems. Nearly 80% of patients had at least one adverse event after two years. Readmissions to the hospital that were not scheduled are common. Patients are readmitted on average 2.2 times over the course of their 11-month median follow-up period. Device thrombosis, bleeding issues, renal impairment, ischemic and hemorrhagic strokes, multi-organ failure, and infections are all major non-surgical adverse events and consequences linked with LVAD. If left untreated, certain problems can be fatal to the victims.
Heart Pump Device Market: Opportunities
Development of non-invasive, portable, and advanced fetal monitors to bring growth opportunities for global market
Various companies with items in the development phase make up the devices market. Companies with products in the pipeline concentrate on finding unique solutions that will help the market grow faster. For example, the TAH industry is a fast-paced area of device development that is expected to have a rise in launches and approvals in the future years. When compared to the SynCardia Total Heart (sole player until December 2020), these TAHs offer more sophisticated functions, making them a better alternative in the future. CARMAT's entire artificial heart, for example, got the CE Mark in December 2020. CARMAT's TAH is self-regulating, changing blood flow in response to the patient's physical activity.
Heart Pump Device Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Heart Pump Device Market |
| Market Size in 2021 | USD USD 1983.10 Billion |
| Market Forecast in 2028 | USD USD 6162.34 Billion |
| Growth Rate | CAGR of 20.8% |
| Number of Pages | 110 |
| Key Companies Covered | Abbott Laboratories (US), Abiomed (US), Medtronic (Ireland), Teleflex Incorporated (US), SynCardia Systems (US), Fresenius Medical Care AG & Co. KGaA (Germany), Getinge (Sweden), CardiacAssist, Inc. (US), Berlin Heart (Germany), Jarvik Heart, Inc. (US), CARMAT (France), SENKO MEDICAL INSTRUMENT Mfg. CO., LTD. (Japan), Angiodroid (Italy), CardioDyme (US), World Heart Corporation (US)., |
| Segments Covered | By Product, By Type, By Therapy and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2021 |
| Historical Year | 2016 to 2020 |
| Forecast Year | 2022 - 2028 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Global Heart Pump Device Market: Segmentation
The global Heart Pump Device market is segregated based on product ,type,therapy and region.
The heart pump devices market is divided into three categories based on products: ventricular assist devices, intra-aortic balloon pumps, and whole artificial hearts. The largest and fastest-growing segment of this market is ventricular assist devices. Technological developments, a scarcity of organ donors, and an increase in the global prevalence of heart failure are all contributing to this segment's growth.
The market is divided into implanted heart pump devices and extracorporeal heart pump devices based on type. Due to the introduction of innovative products by major players and the increased need for an efficient solution to manage heart failure, implantable heart pump devices will account for the highest share of this market by 2020.
The market is divided into bridge-to-transplant (BTT), bridge-to-candidacy (BTC), destination treatment (DT), and other therapies, depending on the therapy. The BTT segment held the greatest proportion of this market in 2020. The rise is the segment is due to the availability of VADs for BTT and increased awareness of transplantation
Recent Developments
- February 2021 - The FDA has given CARMAT permission to perform an Early Feasibility Study (EFS) of its TAH in the United States.
- December 2020 - The amended labeling for Abbott's Heart Mate 3 Heart Pump has been approved by the FDA for use in juvenile patients with advanced refractory left ventricular heart failure.
Heart Pump Device Market: Regional Landscape
The market for heart pump devices is dominated by the Americas, owing to an increase in the frequency of cardiovascular disorders and an ageing population. Moreover, the government's increased initiatives, as well as funding for research & development in innovative medical treatment alternatives, will fuel the expansion of the heart pump devices market in the region throughout the forecast period. Due to an increase in the frequency of chronic cardiovascular illnesses and rising healthcare costs, Asia-Pacific is expected to see considerable growth in the heart pump devices market. Furthermore, the region's improving standard of living is expected to fuel the expansion of the heart pump devices market in the future years.
Heart Pump Device Market: Competitive Landscape
Some of the main competitors dominating the global Heart Pump Device market include -
- Abbott Laboratories (US)
- Abiomed (US)
- Medtronic (Ireland)
- Teleflex Incorporated (US)
- SynCardia Systems (US)
- Fresenius Medical Care AG & Co. KGaA (Germany)
- Getinge (Sweden)
- CardiacAssist
- Inc. (US)
- Berlin Heart (Germany)
- Jarvik Heart
- Inc. (US)
- CARMAT (France)
- SENKO MEDICAL INSTRUMENT Mfg. CO.
- LTD. (Japan)
- Angiodroid (Italy)
- CardioDyme (US)
- World Heart Corporation (US).
Global Heart Pump Device market is segmented as follows:
By Product
- Ventricular Assist Devices
- Intra aortic Balloon Pumps
- Total Artificial Hearts
By Type
- Implanted Heart Pump Device
- Extracorporeal Heart Pump Device
By Therapy
- Bridge-to-Transplant (BTT)
- Bridge-to-Candidacy (BTC)
- Destination Therapy (DT)
- Other Therapies
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
The need for heart pump devices is being driven by an increase in the prevalence of cardiovascular disorders, an increase in the number of regulatory approvals, and a large gap between the demand and availability of donor hearts. The rising reliance on ventricular assist devices for the treatment of heart failure, as well as technological improvements to improve the safety of these devices, are driving the ventricular assist devices market. During the projection period, the high cost of devices and implantation procedures, as well as the poor reimbursement situation in developing countries, are projected to limit the market's growth.
According to the Market Research report, the global Heart Pump Device market was worth about 1983.10 (USD billion) in 2021 and is predicted to grow to around 6162.34 (USD billion) by 2028, with a compound annual growth rate (CAGR) of around 20.80 percent.
Due to an increase in the prevalence of cardiovascular illnesses and an ageing population, the Americas dominate the market for heart pump devices. Furthermore, the government's increasing initiatives, as well as financing for research and development in breakthrough medical treatment alternatives, will propel the heart pump devices market in the region to new heights over the forecast period. Asia-Pacific is predicted to witness significant growth in the heart pump devices market due to an increase in the prevalence of chronic cardiovascular disorders and growing healthcare costs. In addition, the growing standard of living in the region is likely to boost the growth of the heart pump devices market in the coming years.
Some of the main competitors dominating the global Heart Pump Device market include - Abbott Laboratories (US), Abiomed (US), Medtronic (Ireland), Teleflex Incorporated (US), SynCardia Systems (US), Fresenius Medical Care AG & Co. KGaA (Germany), Getinge (Sweden), CardiacAssist, Inc. (US), Berlin Heart (Germany), Jarvik Heart, Inc. (US), CARMAT (France), SENKO MEDICAL INSTRUMENT Mfg. CO., LTD. (Japan), Angiodroid (Italy), CardioDyme (US), and World Heart Corporation (US).
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed