Hemophilia B Gene Therapy Market Size, Share, Analysis, Growth, 2032

Hemophilia B Gene Therapy Market By Patient Age Group (Geriatric, Adults, and Pediatric), By Disease Severity (Severe Hemophilia B, Moderate Hemophilia B, and Mild Hemophilia B), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2024 - 2032
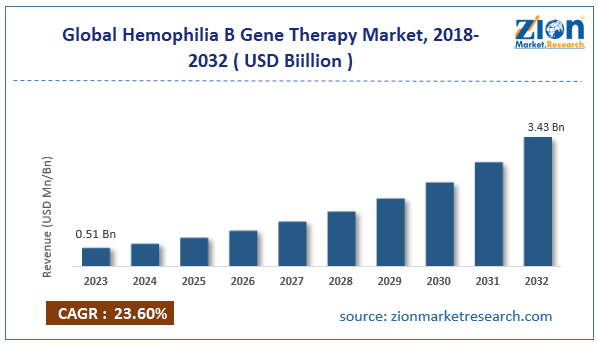
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 0.51 Billion | USD 3.43 Billion | 23.60% | 2023 |
Hemophilia B Gene Therapy Industry Perspective:
The global hemophilia B gene therapy market size was worth around USD 0.51 billion in 2023 and is predicted to grow to around USD 3.43 billion by 2032, with a compound annual growth rate (CAGR) of roughly 23.60% between 2024 and 2032.
Hemophilia B Gene Therapy Market: Overview
Hemophilia B gene therapy is a one-time novel medical treatment for patients with hemophilia B medical conditions. Patients suffering from the illness inherit gene mutation, resulting in the body's inability to produce sufficient levels of a crucial blood clotting factor called factor IX.
Thus, patients with hemophilia B are at the risk of life-threatening bleeding, which can either occur in case of a trauma or spontaneously. In addition to this, everyday activities can also trigger prolonged bleeding internally or externally, further putting the lives of patients at risk. The most common occurrences of internal bleeding may include muscles or joints. The currently more prevalent method of hemophilia B treatment is regular intravenous infusions of factor IX protein. In gene therapy, medical professionals aim to deliver long-term treatment for the serious medical condition. Gene therapy-based one-time infusion leverages the performance of a vector to deliver the required functional genes or focus on replacing the defective gene in the patient.
After the treatment, the patients are expected to show protection against bleeding. Hemophilia B gene therapy is a relatively new medical treatment and faces challenges in terms of reaching a broader audience. However, increased healthcare investments worldwide and growing awareness about the medical condition are projected to help the hemophilia B gene therapy industry thrive.
Key Insights:
- As per the analysis shared by our research analyst, the global hemophilia B gene therapy market is estimated to grow annually at a CAGR of around 23.60% over the forecast period (2024-2032)
- In terms of revenue, the global hemophilia B gene therapy market size was valued at around USD 0.51 billion in 2023 and is projected to reach USD 3.43 billion by 2032.
- The hemophilia B gene therapy market is projected to grow at a significant rate due to the growing number of patients with hemophilia B
- Based on patient age group, the adult segment is growing at a high rate and will continue to dominate the global market as per industry projections.
- Based on severity, the severe hemophilia B segment is anticipated to command the largest market share.
- Based on region, North America is projected to dominate the global market during the forecast period.
Hemophilia B Gene Therapy Market: Growth Drivers
Growing number of patients with hemophilia B to drive market demand rate
The global hemophilia B gene therapy market is expected to grow due to the rising number of patients suffering from the medical condition. Hemophilia B is a hereditary medical condition caused by the absence of blood clotting factor IX. In case of bleeding, the body undergoes a series of chemical reactions that assist in the formation of blood clots. The process is called coagulation cascade and is a result of 20 different special proteins known as clotting factors working together to prevent excessive bleeding. One such clotting factor is Factor IX. Hemophilia B is caused if the body does not produce enough factor IX.
According to healthcare experts, the condition is an inherited X-linked recessive trait. X chromosome, in the case of hemophilia B, consists of a variant gene. Further market analysis suggests that women with variant factor IX genes are called carriers. Boys being born of carrier women have a 50% chance of having hemophilia B, while daughters have a 50% chance of being a carrier in the future. The major risk factors for hemophilia B include being male and having a family history of bleeding. The main symptom of hemophilia B is prolonged bleeding, which can be first seen in case an infant is circumcised.
Other bleeding-related problems may show up when an infant starts crawling or walking. Some other main symptoms include bruising, blood in stool or urine, bleeding into joints, swelling and pain, and bleeding that may start without a cause. According to the Centers for Disease Control and Prevention (CDC), around 33,000 US males live with hemophilia B.
Ongoing investments in healthcare infrastructure and a surge in patient awareness will aid market expansion
The growing world population and burgeoning pressure on medical care architecture worldwide have promoted new waves of strategic investments that aim to improve healthcare access for the global population. These investments are carried out by government bodies and private companies that are focusing on optimizing the healthcare resources currently at their disposal while creating growth avenues for new treatments and point-of-care. Such measures are likely to promote growth in the global hemophilia B gene therapy market.
For instance, in December 2024, the European Investment Bank (EIB) announced a financing of EUR 35 million to GVN, encouraging the latter to invest in research & development (R&D) and digitalization of the healthcare sector.
Hemophilia B Gene Therapy Market: Restraints
Lack of access to the treatment to limit the industry’s growth rate during the forecast period
The global industry for hemophilia B gene therapy is projected to be restricted by the lack of access to the novel treatment. According to market analysis, gene therapy for hemophilia B is a relatively new treatment. In addition, gene therapy for hemophilia has not been accepted on a global scale and only a few countries permit the use of the treatment method. The high cost associated with gene therapy research and treatment, along with the lack of standard regulatory protocols, may impact overall revenue in the market.
Hemophilia B Gene Therapy Market: Opportunities
Ongoing research and innovation in gene therapy for the blood clotting disorder will create expansion possibilities
The global hemophilia B gene therapy market is projected to generate growth opportunities due to the increasing research & development in the industry. For instance, in April 2024, the US Food & Drugs Administration (FDA) announced the approval of BEQVEZ™ (fidanacogene elaparvovec-dzkt). It is a one-time gene therapy for treating hemophilia B in adults.
According to official reports, a one-time dose of the new medicine has significantly reduced bleeds post-treatment. BEQVEZ™ is produced by leading pharmaceutical giant Pfizer, and it can replace prophylaxis therapy in patients with mild to severe hemophilia B. According to the claims of the pharmaceutical giant, the novel medicine is an adeno-associated virus (AAV)-based gene therapy. It is designed to deliver a highly functional copy of the FIX gene in transduced cells.
In November 2022, the US FDA approved etranacogene dezaparvovec (Hemgenix), marking a landmark event in gene therapy and hematology. On the other hand, in June 2024, the National Institute for Health and Care Excellence (NICE), a UK-based public body focusing on social care and regional health infrastructure, announced the approval of Hemgenix® to be used for treating hemophilia B in England.
Hemophilia B Gene Therapy Market: Challenges
Side-effects associated with gene therapy to challenge market expansion
The global hemophilia B gene therapy industry is projected to be challenged by the prevalence of several side effects after undergoing gene therapy. For instance, the short-term side effects include allergic reactions, flu-like symptoms, stomach discomfort, and rash. On the other hand, more serious side reactions include inflamed liver, increased risk of cancer, and blood clots. Gene therapy may not deliver optimal results in all patients, which limits the industry’s application rate.
 Request Free Sample
Request Free Sample
Hemophilia B Gene Therapy Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Hemophilia B Gene Therapy Market |
| Market Size in 2023 | USD 0.51 Billion |
| Market Forecast in 2032 | USD 3.43 Billion |
| Growth Rate | CAGR of 23.60% |
| Number of Pages | 225 |
| Key Companies Covered | Freeline Therapeutics, CSL Behring, Alnylam Pharmaceuticals, Dimension Therapeutics, Bayer AG, Pfizer Inc., Sangamo Therapeutics, Roche Holding AG, Spark Therapeutics, Genethon, uniQure N.V., REGENXBIO Inc., BioMarin Pharmaceutical Inc., Novo Nordisk A/S, Shire plc (now part of Takeda Pharmaceutical Company), and others. |
| Segments Covered | By Patient Age Group, By Disease Severity, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Hemophilia B Gene Therapy Market: Segmentation
The global hemophilia B gene therapy market is segmented based on patient age group, disease severity, and region.
Based on the patient age group, the global market segments are geriatric, adults, and pediatric. In 2023, the highest growth was listed in the adult segment. According to market research, currently available gene therapy treatments are mostly studied for adults.
In addition, patient groups in the adult segment have shown a higher tendency to respond well to gene therapy treatment, which has helped the segment thrive. However, ongoing research on hemophilia B in children will help the pediatric segment grow in the coming years. Hemophilia B is known to affect 1 in 25,000 to 30,000 male births.
Based on the severity, the hemophilia B gene therapy industry divisions are severe hemophilia B, moderate hemophilia B, and mild hemophilia B. In 2023, the highest demand was listed in the severe hemophilia B segment. According to official estimates, nearly 60.01% of all hemophilia B cases are severe. The patients contain around 1% of less than normal levels of factor IX. In addition to this, the majority of treatment developers are focusing on developing treatments for severe forms. Mild hemophilia b is observed in nearly 25% to 30% of all cases.
Hemophilia B Gene Therapy Market: Regional Analysis
North America to continue leading the overall growth rate during the forecast period
The global hemophilia B gene therapy market is expected to be dominated by North America in the coming years. The US will lead the regional revenue due to the presence of one of the world’s most advanced research sectors focusing on developing new treatments for hemophilia B. The US market is more mature compared to other regional territories.
In addition to this, awareness around hemophilia B, including its causes and possible treatments, is widely available across major official sites, allowing enhanced patient awareness. Furthermore, the regional pharmaceutical giants have increased spending on gene therapy-based treatments as the novel of medical care has shown exceptional results in treating rare disorders.
Europe is expected to emerge as the significant player driving the demand for gene therapies for treating hemophilia B. The growing number of patients suffering from the life-altering bleeding disorder across European nations will create more demand for effective treatments. In addition, the presence of a widely accessible and cost-effective healthcare infrastructure in Germany, France, the UK, and Spain will allow better adoption of gene therapy for treating patients of hemophilia B.
Hemophilia B Gene Therapy Market: Competitive Analysis
The global hemophilia B gene therapy market is led by players like:
- Freeline Therapeutics
- CSL Behring
- Alnylam Pharmaceuticals
- Dimension Therapeutics
- Bayer AG
- Pfizer Inc.
- Sangamo Therapeutics
- Roche Holding AG
- Spark Therapeutics
- Genethon
- uniQure N.V.
- REGENXBIO Inc.
- BioMarin Pharmaceutical Inc.
- Novo Nordisk A/S
- Shire plc (now part of Takeda Pharmaceutical Company)
The global hemophilia B gene therapy market is segmented as follows:
By Patient Age Group
- Geriatric
- Adults
- Pediatric
By Disease Severity
- Severe Hemophilia B
- Moderate Hemophilia B
- Mild Hemophilia B
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Hemophilia B gene therapy is a one-time novel medical treatment used for treating patients with hemophilia B medical conditions.
The global hemophilia B gene therapy market is expected to grow due to the rising number of patients suffering from the medical condition.
According to study, the global hemophilia B gene therapy market size was worth around USD 0.51 billion in 2023 and is predicted to grow to around USD 3.43 billion by 2032.
The CAGR value of the hemophilia B gene therapy market is expected to be around 23.60% during 2024-2032.
The global hemophilia B gene therapy market is expected to be dominated by North America in the coming years.
The global hemophilia B gene therapy market is led by players like Freeline Therapeutics, CSL Behring, Alnylam Pharmaceuticals, Dimension Therapeutics, Bayer AG, Pfizer Inc., Sangamo Therapeutics, Roche Holding AG, Spark Therapeutics, Genethon, uniQure N.V., REGENXBIO Inc., BioMarin Pharmaceutical Inc., Novo Nordisk A/S and Shire plc (now part of Takeda Pharmaceutical Company).
The report explores crucial aspects of the hemophilia B gene therapy market, including a detailed discussion of existing growth factors and restraints while also browsing future growth opportunities and challenges that impact the market.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed