HIV/AIDS Diagnostics Market Size, Share, Trends, Growth, Forecasts, 2032

HIV/AIDS Diagnostics Market By Product type (instruments, kits and reagents, and others), By Test type (nucleic acid tests, rapid tests (POC), ELISA, and others), By End-user (private diagnostics laboratories, hospitals, research institutes & others, and academic) And By Region: - Global And Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, And Forecasts, 2024-2032
| Market Size in 2023 | Market Forecast in 2032 | Growth Rate (in %) | Base Year |
|---|---|---|---|
| USD 4,583.90 Million | USD 10,897.40 Million | CAGR at 10.10% | 2023 |
Description
Global HIV/AIDS Diagnostics Market: Insights
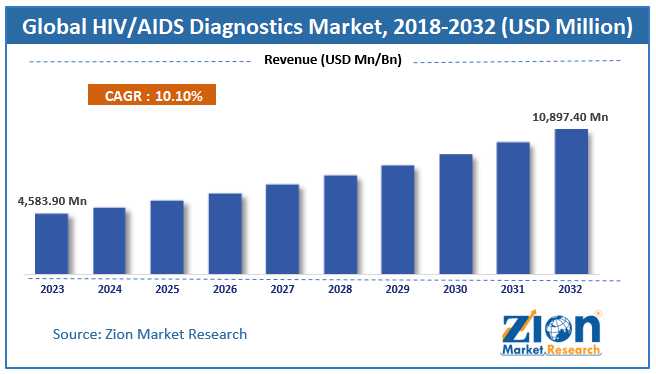
According to the report published by Zion Market Research, the global HIV/AIDS Diagnostics Market size was valued at USD 4,583.90 Million in 2023 and is predicted to reach USD 10,897.40 Million by the end of 2032. The market is expected to grow with a CAGR of 10.10% during the forecast period. The report analyzes the global HIV/AIDS Diagnostics Market’s growth drivers, restraints, and impact on demand during the forecast period. It will also help navigate and explore the arising opportunities in the HIV/AIDS Diagnostics Market industry.
Global HIV/AIDS Diagnostics Market: Overview
HIV/AIDS diagnostics are used to examine the presence of the human immune-deficiency virus (HIV), the virus that causes acquired immunodeficiency syndrome (AIDS), in saliva, serum or urine. HIV/AIDS diagnostics helps to detect antibodies, RNA or antigens. Blood tests are the most regular way to make a diagnosis of HIV. HIV is most commonly diagnosed by doing a urine test and blood test.
Global HIV/AIDS Diagnostics Market: Growth Factors
According to WHO research, until now around 75 million people are infected with human immune-deficiency virus (HIV) and around 30 million people died of HIV disease. With the growing mortality rate, the government and non-government organizations are taking major initiatives to increase the awareness among the people regarding HIV/AIDS and are also providing economic help for diagnostic management thus driving the HIV/AIDS diagnostics market. With the progression of technology, novel diagnostic products are easily accessible in the market which needs the least amount of technical skills that fasten the diagnosis process.
In developing countries such as China and India where a growing population is becoming a major concern, increasing awareness about HIV, and up-coming economies will be the major driving factor for HIV/AIDS diagnostics market. Rise in incidence of HIV infection, knowledge about HIV/AIDs diagnosis and treatment, growing research & development for treatment, early diagnosis, and prevention are the other major factors boosting the HIV/AIDS diagnostics market.
Key Insights
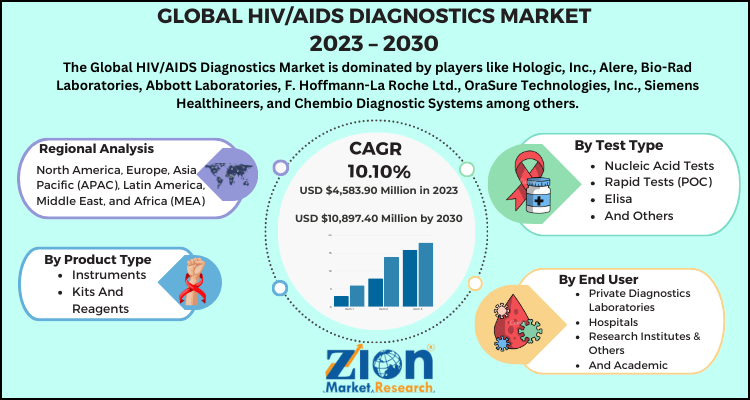
- As per the analysis shared by our research analyst, the global HIV/AIDS Diagnostics Market is estimated to grow annually at a CAGR of around 10.10% over the forecast period (2024-2032).
- In terms of revenue, the global HIV/AIDS Diagnostics Market size was valued at around USD 4,583.90 Million in 2023 and is projected to reach USD 10,897.40 Million by 2032.
- Based on the roduct type, consumables this segment encompassing reagents, assays, and test kits, holds the largest market share due to the recurring need for these items in diagnostic procedures and the introduction of rapid and point-of-care (POC) test kits.
- Based on the test type, antibody tests including ELISA/EIA and rapid tests, dominate the market with a 56.6% share in 2024. Their high sensitivity and widespread use in HIV-1 screening and confirmation contribute to their leading position.
- Based on the end-user, thr diagnostic laboratories accounted for the largest share of the HIV diagnostics market. This dominance is attributed to the rising prevalence of HIV/AIDS, the growing number of diagnostic laboratories, and advancements in point-of-care (POC) instruments.
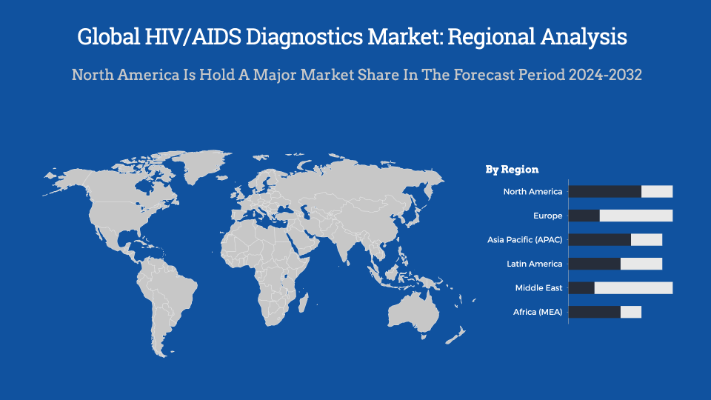
- Based on the region, the North America leads the global HIV diagnostics market, driven by a well-established healthcare infrastructure, high awareness of HIV/AIDS, and strong government initiatives aimed at early detection and prevention.
HIV/AIDS Diagnostics Market: Dynamics
Key Growth Drivers
The HIV/AIDS diagnostics market is primarily driven by the rising prevalence of HIV infections globally, leading to a growing demand for accurate and early diagnosis. Increased awareness programs, government initiatives, and non-governmental organization (NGO) efforts to combat HIV/AIDS are significantly contributing to market growth. Advances in diagnostic technologies, such as rapid diagnostic tests (RDTs), nucleic acid tests (NATs), and point-of-care (POC) testing, offer faster and more reliable results, enhancing early detection and treatment. Additionally, the increasing availability of diagnostic facilities in emerging economies, coupled with expanded access to antiretroviral therapy (ART), further supports market growth.
Restraints
Despite the positive developments, the market faces several restraints, including the high cost of advanced diagnostic tests and limited accessibility in low-income regions. Many developing countries struggle with inadequate healthcare infrastructure, limiting the availability of sophisticated diagnostic tools. Furthermore, the social stigma and discrimination associated with HIV/AIDS often discourage individuals from seeking timely testing, thereby impacting market growth. Regulatory challenges and lengthy approval processes for new diagnostic products also create barriers for manufacturers aiming to introduce innovative technologies.
Opportunities
The increasing focus on early diagnosis and treatment to achieve the global goal of ending the HIV epidemic presents lucrative opportunities for the market. Technological advancements in diagnostic assays and the development of portable and user-friendly testing devices are creating new growth avenues. Expansion of community-based testing programs and self-testing kits offers accessible and convenient diagnostic options, particularly in remote and underserved areas. Moreover, public-private partnerships and international funding for HIV programs are driving investments in diagnostic infrastructure and research, supporting market expansion.
Challenges
A major challenge in the HIV/AIDS diagnostics market is ensuring equitable access to diagnostic services, particularly in resource-limited settings. Many regions face shortages of trained healthcare professionals and inadequate laboratory facilities, hampering effective testing and diagnosis. Additionally, maintaining the quality and accuracy of diagnostic results in decentralized and community settings remains a concern. The ongoing mutation of the HIV virus and the emergence of drug-resistant strains also pose diagnostic challenges, requiring continuous advancements in test sensitivity and specificity. Furthermore, sustaining funding for HIV programs amid competing healthcare priorities can impact market growth in certain regions.
HIV/AIDS Diagnostics Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | HIV/AIDS Diagnostics Market Research Report |
| Market Size in 2023 | USD 4,583.90 Million |
| Market Forecast in 2032 | USD 10,897.40 Million |
| Growth Rate | CAGR of 10.10% |
| Number of Pages | 196 |
| Key Companies Covered | Hologic, Inc., Alere, Bio-Rad Laboratories, Abbott Laboratories, F. Hoffmann-La Roche Ltd., OraSure Technologies, Inc., Siemens Healthineers, and Chembio Diagnostic Systems among others |
| Segments Covered | By Product Type, By Test Type, By End-user And By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Global HIV/AIDS Diagnostics Market: Segmentation
The HIV/AIDS diagnostics market is divided based on the product type, test type, and end-user. All the segments have been analyzed based on present and future trends and the market is estimated from 2024 to 2032.
Based on the product type, the HIV/AIDS diagnostics market has been classified into instruments, kits and reagents, and others.
Based on the test type, the HIV/AIDS diagnostics market is categorized into nucleic acid tests, rapid tests (POC), ELISA, and others.
Based on the end users, the global HIV/AIDS diagnostics market is divided into private diagnostics laboratories, hospitals, research institutes & others, and academic.
The regional segment includes the current and forecast demand for North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa.
Global HIV/AIDS Diagnostics Market: Regional Analysis
The HIV/AIDS diagnostics market is dominated by North America, particularly the United States, due to advanced healthcare infrastructure, high awareness, robust government initiatives, and significant investments in diagnostic technologies. According to recent data (2023-2024), North America holds the largest market share (~40%), driven by widespread screening programs, high adoption of advanced testing (molecular diagnostics, rapid tests), and strong regulatory support (CDC, FDA). Europe follows closely (~30%), with growth fueled by increasing testing rates and government-funded HIV programs.
Meanwhile, the Asia-Pacific region is the fastest-growing market (~20% CAGR), owing to rising prevalence, improving healthcare access, and expanding diagnostic campaigns in countries like India and China. Africa, despite high HIV burden, has a smaller market share (~10%) due to limited healthcare spending but remains critical for rapid test adoption. North America’s dominance is further reinforced by key industry players (Abbott, Roche, Siemens) and high R&D investments in novel diagnostics.
Global HIV/AIDS Diagnostics Market: Competitive Players
Some of the major companies in the global HIV/AIDS diagnostics market are:
- Hologic, Inc.
- Alere, Bio-Rad Laboratories
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd.
- OraSure Technologies, Inc.
- Siemens Healthineers
- Chembio Diagnostic Systems among others.
The Global HIV/AIDS Diagnostics Market is segmented as follows:
By Product Type
- Instruments
- Kits And Reagents
- And Others
By Test Type
- Nucleic Acid Tests
- Rapid Tests (POC)
- ELISA
- And Others
By End-user
- Private Diagnostics Laboratories
- Hospitals
- Research Institutes & Others
- And Academic
Global HIV/AIDS Diagnostics Market: Regional Segment Analysis
- North America
- The U.S.
- Europe
- The UK
- France
- Germany
- The Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- The Middle East and Africa
What Reports Provides
- Full in-depth analysis of the parent market
- Important changes in market dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional markets
- Testimonials to companies in order to fortify their foothold in the market.
Table Of Content
FrequentlyAsked Questions
HIV/AIDS diagnostics are used to examine the presence of the human immune-deficiency virus (HIV), the virus that causes acquired immunodeficiency syndrome (AIDS), in saliva, serum or urine. HIV/AIDS diagnostics helps to detect antibodies, RNA or antigens. Blood tests are the most regular way to make a diagnosis of HIV. HIV is most commonly diagnosed by doing a urine test and blood test.
The HIV/AIDS Diagnostics Market was valued at USD 4,583.90 Million in 2023.
The HIV/AIDS Diagnostics Market is expected to reach USD 10,897.40 Million by 2032, growing at a CAGR of of 10.10% between 2024 to 2032.
According to WHO research, until now around 75 million people are infected with human immune-deficiency virus (HIV) and around 30 million people died of HIV disease. With the growing mortality rate, the government and non-government organizations are taking major initiatives to increase the awareness among the people regarding HIV/AIDS and are also providing economic help for diagnostic management thus driving the HIV/AIDS diagnostics market. With the progression of technology, novel diagnostic products are easily accessible in the market which needs the least amount of technical skills that fasten the diagnosis process.
HIV/AIDS Diagnostics Market players such as Hologic, Inc., Alere, Bio-Rad Laboratories, Abbott Laboratories, F. Hoffmann-La Roche Ltd., OraSure Technologies, Inc., Siemens Healthineers, and Chembio Diagnostic Systems among others.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed