Immuno-Oncology Clinical Trials Market Size, Global Analysis Report 2032

Immuno-Oncology Clinical Trials Market- By Design (Interventional Trials and Observational Trials), By Phase (Phase I, Phase II, Phase III, and Phase IV), By Indication (Solid Tumors and Hematological Cancer), And By Region- Global Industry Perspective, Comprehensive Analysis, and Forecast, 2024 - 2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 8.35 Billion | USD 26.84 Billion | 12.4% | 2023 |
Immuno-Oncology Clinical Trials Market Insights
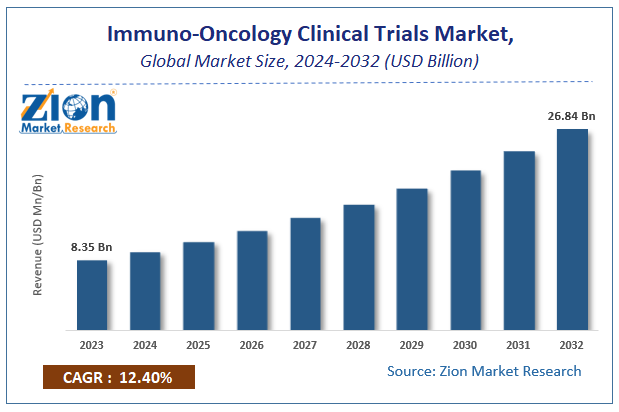
According to Zion Market Research, the global Immuno-Oncology Clinical Trials Market was worth USD 8.35 Billion in 2023. The market is forecast to reach USD 26.84 Billion by 2032, growing at a compound annual growth rate (CAGR) of 12.4% during the forecast period 2024-2032.
The report offers a comprehensive market analysis, highlighting the factors that will determine growth, potential challenges, and opportunities that could emerge in the Immuno-Oncology Clinical Trials Market industry over the next decade.
Immuno-oncology Clinical Trials Market: Synopsis
Design and analysis of immuno-oncology clinical trials have been driven by efficiency patterns and toxicity observed in chemotherapy as well as other targeted agents. This, in turn, helps in completely informing clinical trial design as well as guiding therapeutic decision-making in immuno-oncology. Breakthroughs in immuno-oncology have brought a paradigm shift in cancer therapies and drug development and this is likely to crop up the need for carrying out more immuno-oncology clinical trials. However, there has been a big task for conducting these trials due to the rapidly emerging landscape of the healthcare industry.
Apart from this, the design & analysis of these clinical trials is facing huge challenges in terms of non-proportional hazard patterns translated into Kaplan-Meier curves encompassed by end-points referred as a time to event end-points. Furthermore, researchers have devised new immunotherapy strategies for addressing concerns about the failure of immuno-oncology clinical trials, thereby paving the way for the expansion of the immuno-oncology clinical trials market. Moreover, breakthroughs in immuno-oncology clinical trial strategies have resulted in the development of effective drugs for treating immuno-oncology disorders.
Furthermore, immune checkpoint inhibitors have strongly impacted the oncology domain resulting in a spike in clinical trial enrollment for clinical trials supported by a rise in its approval by authorities such as the FDA. For the record, in 2022, there were over 900 immuno-oncology clinical tests performed, and more than 1,75,000 patients enrolled for clinical trials on myriad kinds of combinations of immuno-oncology drugs.
Immuno-oncology Clinical Trials Market: Growth Drivers
With immunotherapy adding a new dimension to cancer treatment and becoming a major pillar in oncotherapeutics armamentarium, the market for Immuno-oncology Clinical Trials is likely to gain traction in years to come. Massive acceptance of targeted drug treatment methods and breakthroughs in immuno-oncology will shape the growth sphere of the Immuno-oncology Clinical Trials industry in the ensuing years. Furthermore, immuno-oncology agents harvest exceptional anti-tumor mechanisms and favorably influence host immune systems, thereby resulting in positive immune responses. Moreover, these aspects have led to optimal integration of immunotherapy into immuno-oncology clinical trials. This is likely to enlarge the scope of growth for the immuno-oncology clinical trials industry in the span of the next few years.
Reportedly, immuno-oncology clinical trials contribute nearly over one-third of total oncology clinical trials, thereby playing a major role in impacting returns for the immuno-oncology clinical trials market. The rise in the incidence of cancer has forced biotech & pharma firms to carry out more immuno-oncology clinical tests, thereby accounting for the major chunk of the revenue share of the immuno-oncology clinical trials market.
Immuno-Oncology Clinical Trials Market: Regional Analysis
- North American Market To Reach Highest Peak Of Growth By 2032
The expansion of the immuno-oncology clinical trials market in North America over the forecast timespan can be credited to a surge in demand for personalized drugs to be used in new cancer treatments. In addition to this, large budget allocation for cancer research activities by government as well as private firms is expected to lead to increment in number of immuno-oncology clinical trials in sub-continent. This is likely to augment market profitability in the region.
For the record, North America accounted for nearly 52% of the overall market revenue share in 2023 and is projected to continue its major contributions toward overall market proceeds even in the next seven years.
Immuno-Oncology Clinical Trials Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Immuno-Oncology Clinical Trials Market |
| Market Size in 2023 | USD 8.35 Billion |
| Market Forecast in 2032 | USD 26.84 Billion |
| Growth Rate | CAGR of 12.4% |
| Number of Pages | 187 |
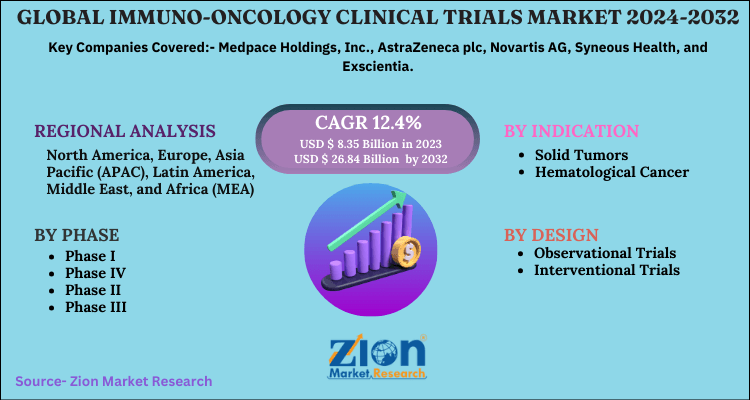
| Key Companies Covered | Medpace Holdings, Inc., AstraZeneca plc, Novartis AG, Syneous Health, and Exscientia |
| Segments Covered | By Design, By Phase, By Indication, And By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Immuno-Oncology Clinical Trials Market: Competitive Analysis
Some of the major players in the global Immuno-Oncology Clinical Trials market include:
- Medpace Holdings Inc.
- AstraZeneca plc
- Novartis AG
- Syneous Health
- Exscientia.
The global Immuno-Oncology Clinical Trials Market is segmented as follows:
By Design
- Observational Trials
- Interventional Trials
By Phase
- Phase I
- Phase IV
- Phase II
- Phase III
By Indication
- Solid Tumors
- Hematological Cancer
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
With immunotherapy adding a new facet to cancer treatment and becoming a major tool in oncotherapeutics armamentarium, the market for Immuno-oncology Clinical Trials is likely to expand in years to come. Massive acceptance of targeted drug treatment methods and breakthroughs in immuno-oncology will shape the growth dimensionality of the Immuno-oncology Clinical Trials industry in forthcoming years.
According to a study, the global Immuno-Oncology Clinical Trials market size was worth around USD 8.35 billion in 2023 and is expected to reach USD 26.84 billion by 2032.
North America is expected to dominate the Immuno-Oncology Clinical Trials market over the forecast period.
The key market participants include Medpace Holdings, Inc., AstraZeneca plc, Novartis AG, Syneous Health, and Exscientia.
Choose License Type
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed