In Vitro Diagnostics (IVD) Market Size, Global Trends & Forecast 2030

In Vitro Diagnostics (IVD) Market By Product & Service (Reagents, Instruments, and Software & Services), By Technique (Immunodiagnostics, Hematology, Molecular Diagnostics, Tissue Diagnostics, and Clinical Chemistry), By Application (Infectious Disease, Diabetes, Cardiology, Oncology, Autoimmune Disorders, and Drug Testing), By End-User (Hospitals & Clinics, Laboratories, and Homecare Units), and By Region: Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2022 - 2030
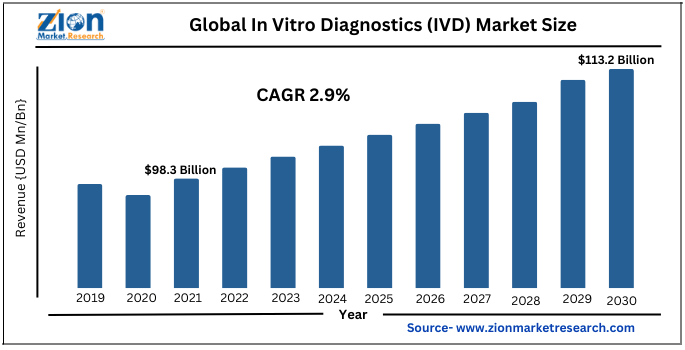
| Market Size in 2021 | Market Forecast in 2028 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 98.3 Billion | USD 117.4 Billion | 2.9% | 2021 |
In Vitro Diagnostics (IVD) Market Size & Industry Perspective:
The Global in vitro diagnostics (IVD) market was valued at $98.3 billion in 2021 and is anticipated to reach $117.4 billion by the end of 2030. The market is projected to grow with a healthy CAGR of 2.9% during the forecast period. The report is a comprehensive study of market definition, growth drivers, opportunities, and challenges. It offers both quantitative and qualitative insights into the global market dynamics.
In Vitro Diagnostics (IVD) Market: Overview
In vitro diagnostic refers to the medical devices that need the help of reagents and assays to diagnose a medical condition. However, these instruments analyze the tissue samples and body fluids collected from patients. These diagnostic systems are widely adopted to execute different in-vitro tests on targeted samples. These are used to diagnose different health conditions like cancer and diabetes. They also monitor different infectious and autoimmune diseases.
Key Insights
- As per the analysis shared by our research analyst, the global In Vitro Diagnostics (IVD) market is estimated to expand annually at a CAGR of around 2.9% over the forecast period (2022-2030)
- In terms of revenue, the global In Vitro Diagnostics (IVD) market was valued at nearly $98.3 billion in 2021 and is anticipated to hit $117.4 billion by 2030
- The market is projected to expand at a significant rate due to growing preference for point of care testing solutions
- Based on product & service, reagents segment accounted for the largest market share in the year 2021
- On basis of technique, molecular diagnostics segment was the leading segment in 2021 and is predicted to record the highest CAGR over the forecast timeframe
- Based on the application, the infectious disease segment is slated to dominate the global market share during the forecast period
- In terms of end-user, hospitals & clinics segment was the leading segment of the global In Vitro Diagnostics (IVD) market in 2021
- On the basis of region, the North American region was the leading revenue generator for the global market in 2021 and will continue to dominate it in the coming years
COVID-19 Impact Analysis:
The outbreak of the Covid-19 pandemic has positively impacted the growth of the global in vitro diagnostics (IVD) market. Due to the severely growing cases of coronavirus, there was high demand for immunodiagnostics and molecular testing, which led to an increase in the sales of these diagnostic kits. There was a significant spike in the volume of point of care testing during the pandemic owing to its capability to do a faster on-site screening. During the pandemic period, several new startups emerged in the market due to the growing scope of in vitro diagnostic kits.
On the contrary, the pandemic has also decreased the demand for in-vitro diagnoses used in autoimmune, nephrology, oncology, and cardiology disorders. This footfall in clinics and healthcare centers led to the decline in the global market. However, with the mixed impact of the Covid-19 on the global market, the market is likely to witness strong growth prospects in the forthcoming years in the global market.
In Vitro Diagnostics (IVD) Market: Growth Drivers
Rapid shift to point-of-care testing is likely to boost the growth of the global market.
The traditional model of laboratory testing all across the globe was a centralized laboratory that utilized automatic analytical testing techniques to detect the target. However, this one was well established in metallurgy discipline & clinical chemistry and is now extending to other spaces also. Point-of-care devices over conventional procedures offer ease of use affordability and yield rapid results that are high in demand for initiating quick decision making. However, the high adoption rate of POC devices is largely impacted by the need to make the healthcare system more patient-centric.
Centralized testing does not offer a convenient process in respect of patients as the testing process is not connected with consultations calls. However, it poses a huge drawback for patients with chronic diseases like diabetes that need constant monitoring. Apart from this, the world is witnessing growing incidences of chronic diseases. Parallelly, there is a rise in awareness among people regarding the early diagnosis and proper treatment, which in turn is further expanding the scope of the global in vitro diagnostics (IVD) market. People became more aware of the availability of advanced novel IVD products.
In Vitro Diagnostics (IVD) Market: Restraints
High cost of IVD instruments is likely to hamper the growth of the global market.
In-vitro diagnostics are quite expensive when compared to other available technology. It also requires constant maintenance of instruments which is further likely to hamper the growth of the global market. Additionally, such instruments also need skilled professionals to operate, thereby limiting its growth.
In Vitro Diagnostics (IVD) Market: Opportunities
The high adoption rate of point of care testing devices is likely to create several lucrative growth opportunities in the global market.
Medical device companies are constantly increasing their research and development capabilities to innovate technologically advanced diagnostic devices. Hospitals and laboratories have shown high interest in the point of care testing devices to collect precise real-time data. Such an increase in interest in point-of-care testing devices is due to the constantly growing burden of diseases on healthcare infrastructure, which requires advanced results in a short period of time. Therefore, such a landscape is likely to offer ample impetus to the growth of the global market in the forthcoming years.
In Vitro Diagnostics (IVD) Market: Challenges
Unfavorable reimbursement landscape is a huge challenge in the global market.
The lack of a proper financing system will hamper the growth of the global market. Advanced treatment is quite expensive, and therefore it restricts people from undergoing treatment through in vitro diagnostics, thereby posing a huge challenge in the global market.
In Vitro Diagnostics (IVD) Market: Segmentation
The global in vitro diagnostics (IVD) market can be segmented into product & service, technique, application, end-user, and region.
By product & service, the global in vitro diagnostics (IVD) industry can be segmented into software & services, instrument, and reagents segments. Moreover, reagents segment is set to account for the largest share of the global market over the forecast timeline. The segmental growth can be attributed to growing demand for quick diagnosis of chronic and genetic disorders in its early stage.
By technique, the global market can be divided into clinical chemistry, tissue diagnostics, molecular diagnostics, hematology, and immunodiagnostics segments. Moreover, the molecular diagnostics segment is predicted to register the fastest growth over the forecast period owing to large-scale use of molecular diagnostics PCR for detecting infection and identifying causative organisms. In comparison to traditional diagnostics techniques, molecular diagnostics PCR techniques target fragments of bacteria to make diagnosis instead of culturable cell tissues.
Apart from this, massive use of molecular diagnostics in identifying fragile X syndrome, the von hippel-lindau disease, cystic fibrosis, glaucoma, age-related macular degeneration, and neurological disorders such as transmissible spongiform encephalopathies & Alzheimer disease will drive the segmental growth.
In terms of application, the global in vitro diagnostics (IVD) market can be bifurcated into Infectious Disease, Diabetes, Cardiology, Oncology, Autoimmune Disorders, and Drug Testing segments. The infectious disease segment is predicted to dominate the global market growth over the forecast timeline subject to massive use of in vitro diagnostics for determining microorganisms in the infectious diseases.
In terms of end-user, the global in vitro diagnostics (IVD) market can be segmented into Hospitals & Clinics, Laboratories, and Homecare Units. The hospitals & clinics segment is predicted to dominate the growth of the global market in the ensuing years as many diagnostic tests are carried out on patients admitted to hospitals.
Recent Developments
- In December 2022, Medsource Ozone Biomedicals, a key Indian player in in vitro diagnostics (IVD) market, launched made in India autoimmune assays. Reportedly, these testing kits will assist the healthcare service providers in early diagnosis of autoimmune disorders. For the record, Medsource is likely to manufacture the autoimmune assays by collaborating with Human Diagnostics GmbH, a key German player in in vitro diagnostics sector. The move will boost the growth of the in vitro diagnostics (IVD) market in India.
- In November 2022, Sentinel Diagnostics, a key Italian manufacturer of in vitro diagnostics reagents, introduced SENTiNAT® 200 and STAT-NAT® SN200 Real-Time PCR assay kits for identifying 10 viruses. Reportedly, these in vitro diagnostic kits are predicted to assist labs in effectively diagnosing viral infections. Such moves are anticipated majorly towards the growth of in vitro diagnostics (IVD) industry across the globe.
In Vitro Diagnostics (IVD) Market Report Scope:
| Report Attributes | Report Details |
|---|---|
| Report Name | In Vitro Diagnostics (IVD) Market Report |
| Market Size in 2021 | USD 98.3 Billion |
| Market Forecast in 2030 | USD 117.4 Billion |
| Compound Annual Growth Rate | CAGR of 2.9% |
| Number of Pages | 261 |
| Forecast Units | Value (USD Billion), and Volume (Units) |
| Key Companies Covered | QIAGEN N.V., Ortho Clinical Diagnostics, DiaSorin, bioMérieux, Sysmex Corporation, Bio-Rad Laboratories, Becton, Dickinson and Company, Johnson & Johnson, Thermo Fisher Scientific, Danaher Corporation, Siemens Healthineers, and Roche Diagnostics. |
| Segments Covered | By Product & Service, By Technique, By Application, By End-User and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East and Africa (MEA) |
| Countries Covered | North America: U.S and Canada Europe: Germany, Italy, Russia, U.K, Spain, France, Rest of Europe APAC: China, Australia, Japan, India, South Korea, South East Asia, Rest of Asia Pacific Latin America: Brazil, Argentina, Chile The Middle East And Africa: South Africa, GCC, Rest of MEA |
| Base Year | 2021 |
| Historical Year | 2016 to 2020 |
| Forecast Year | 2022 - 2030 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
To know more about this report, request a sample copy.
In Vitro Diagnostics (IVD) Market: Regional Landscape
North America accounts for the largest share in the global in vitro diagnostics (IVD) market due to the presence of a robust healthcare system in the region. Furthermore, the high awareness among people in the region regarding the presence of such advanced novel technology is likely to boost the growth of the regional market.
Additionally, the efficient reimbursement policies implemented by the government will further encourage people to adopt such advanced technology to undergo a complete treatment for different diseases, which in turn will accentuate the growth of the regional market.
In Vitro Diagnostics (IVD) Market: Competitive Landscape
Some of the significant players in the global in vitro diagnostics (IVD) market include:
- QIAGEN N.V.
- Ortho Clinical Diagnostics
- DiaSorin
- bioMérieux
- Sysmex Corporation
- Bio-Rad Laboratories
- Becton
- Dickinson and Company
- Johnson & Johnson
- Thermo Fisher Scientific
- Danaher Corporation
- Siemens Healthineers
- Roche Diagnostics.
The global in vitro diagnostics (IVD) market is segmented as follows:
By Product & Service
- Reagents
- Instruments
- Software and Services
By Technique
- Immunodiagnostics
- Hematology
- Molecular Diagnostics
- Tissue Diagnostics
- Clinical Chemistry
By Application
- Infectious Disease
- Diabetes
- Cardiology
- Oncology
- Autoimmune Disorders
- Drug Testing
By End-User
- Hospitals & Clinics
- Laboratories
- Homecare Units
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
The traditional model of laboratory testing all across the globe was a centralized laboratory that utilized automatic analytical testing techniques to detect the target. However, this one was well established in metallurgy discipline and clinical chemistry and is now extending to other spaces also. Parallelly, there is a rise in awareness among people regarding the early diagnosis and proper treatment, which in turn is further expanding the scope of the global in (IVD) market.
The global in vitro diagnostics (IVD) market was valued at $98.3 billion in 2021 and is anticipated to reach $117.4 billion by the end of 2030. The market is projected to grow with a healthy CAGR of 2.9% during the forecast period.
Some of the significant players in the global in vitro diagnostics (IVD) market include QIAGEN N.V., Ortho Clinical Diagnostics, DiaSorin, bioMérieux, Sysmex Corporation, Bio-Rad Laboratories, Becton, Dickinson and Company, Johnson & Johnson, Thermo Fisher Scientific, Danaher Corporation, Siemens Healthineers, and Roche Diagnostics.
North America accounts for the largest share in the global in vitro diagnostics (IVD) market due to the presence of a robust healthcare system in the region. Furthermore, the high awareness among people in the region regarding the presence of such advanced novel technology is likely to boost the growth of the regional market.
Choose License Type
List of Contents
Market Size Industry Perspective:OverviewKey InsightsCOVID-19 Impact Analysis:Growth DriversRestraintsOpportunitiesChallengesSegmentationRecent DevelopmentsMarketReport Scope:To know more about this report,request a sample copy.Regional LandscapeCompetitive LandscapeThe global in vitro diagnostics (IVD) market is segmented as follows:RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed