Medical Device Analytical Testing Outsourcing Market Size, Share, Growth Report 2032

Medical Device Analytical Testing Outsourcing Market By End-use (hospitals and others), By Device type (reprocessed devices and others), By Therapeutic area (diabetes care, dental, endoscopy, drug delivery, general & plastic surgery, ophthalmic, IVD, orthopedic, diagnostic imaging, cardiology testing, and others), By Services (sterility testing, bioburden testing, physical testing, material characterization, and extractable & leachable) And By Region: - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts, 2024-2032
| Market Size in 2023 | Market Forecast in 2032 | Growth Rate (in %) | Base Year |
|---|---|---|---|
| USD 5.55 Billion | USD 11.28 Billion | CAGR at 8.20% | 2023 |
Description
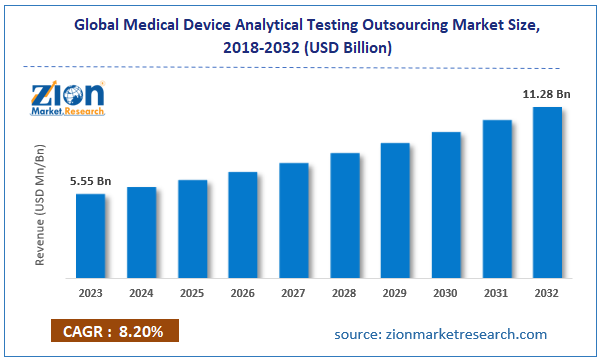
According to the report published by Zion Market Research, the global Medical Device Analytical Testing Outsourcing Market size was valued at USD 5.55 Billion in 2023 and is predicted to reach USD 11.28 Billion by the end of 2032. The market is expected to grow with a CAGR of 8.20% during the forecast period. The report analyzes the global Medical Device Analytical Testing Outsourcing Market ’s growth drivers, restraints, and impact on demand during the forecast period. It will also help navigate and explore the arising opportunities in the Medical Device Analytical Testing Outsourcing Market industry.
Global Medical Device Analytical Testing Outsourcing Market: Overview
Medical device analytical testing outsourcing refers to the process of emphasizing third-party contracts for manufacturing, validation, packaging, prototyping, product designing, and verification of medical devices in a controlled and sterile environment for the supply chain management.
Global Medical Device Analytical Testing Outsourcing Market: Growth Factors
The growing prevalence of chronic diseases across the world is fueling the demand for medical device analytical testing outsourcing. Increasing stringent rules from the government regarding the quality certificate is propelling the growth of the global medical device analytical testing outsourcing market. Class II devices need premarket approvals & 510 (k) clearance, however, getting such clearance is a bit complex process and hence such emerging scenarios have boosted the growth of the global medical device analytical testing outsourcing market.
Medical device manufacturers are adopting consulting services to understand the documentation and regulations to obtain pre-market approvals. The development of advanced devices like surgical microscopes, surgical robots, neurosurgery devices, ophthalmic surgical devices, and many others has minimized the direct contact of humans in the surgical procedure and thus it has become mandatory to get quality certificates for medical devices. The growing demand for minimally invasive surgeries is bolstering the growth of the global medical device analytical testing outsourcing market. However, manufacturers are adopting medical device analytical testing outsourcing to maintain accuracy, precision, quality check, and periodic maintenance to fulfill the required performance.
Global Medical Device Analytical Testing Outsourcing Market: Segmentation
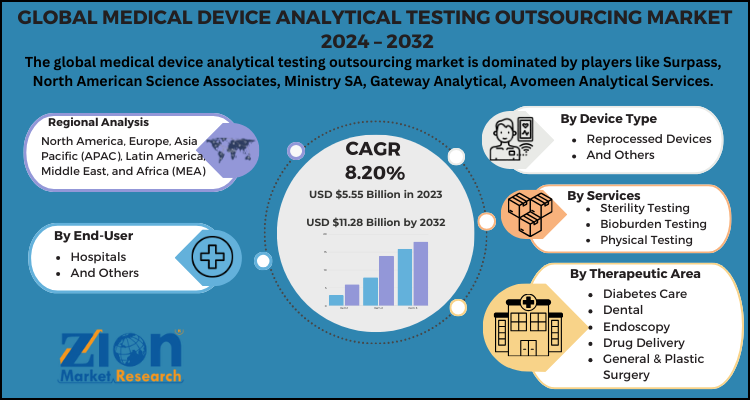
The global medical device analytical testing outsourcing market can be segmented into end-user, device type, therapeutic area, services, and region.
By end-user, the market can be segmented into hospitals and others. The hospital segment holds hegemony over others due to the high budget allocation and patient volume. The hospital segment can further be bifurcated into consumables and equipment. However, other healthcare areas like specialty care centers, ambulatory centers, and diagnostic centers are also expected to grow significantly during the forecast period.
By device types, the market can be segmented into reprocessed devices and others. The analytical testing outsourcing in the other segment dominates the global medical device analytical testing outsourcing market due to the expensive therapeutic and single-user devices.
By therapeutic areas, the market can be segmented into diabetes care, dental, endoscopy, drug delivery, general & plastic surgery, ophthalmic, IVD, orthopedic, diagnostic imaging, cardiology testing, and others. The cardiology testing segment accounts for the largest share in the global medical device analytical testing outsourcing market owing to the complexities in these devices and demand for high-level technical expertise. The general & plastic surgery segment is also anticipated to grow significantly during the forecast period.
By services, the market can be segmented into sterility testing, bioburden testing, physical testing, material characterization, and extractable & leachable.
Medical Device Analytical Testing Outsourcing Market : Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Medical Device Analytical Testing Outsourcing Market Research Report |
| Market Size in 2023 | USD 5.55 Billion |
| Market Forecast in 2032 | USD 11.28 Billion |
| Growth Rate | CAGR of 8.20% |
| Number of Pages | 185 |
| Key Companies Covered | Surpass, BDC Laboratories, NSF International, Source Bioscience, Medical Device Testing Services, Charles River Laboratories, Toxikon, Wuxi Apptec, Pace Analytical Services, North American Science Associates, Ministry SA, Gateway Analytical, Avomeen Analytical Services, Envigo, Elements Material Technology, ASTM, UL, TÜV Rheinland, BSI, DEKRA, TÜV SÜD, Intertek, Bureau Veritas, Eurofins Scientific, SGS, Pharmaceutical Product Development, LLC, Intertek Group PLC, Pace Analytical Services, LLC, West Pharmaceutical Services, Inc., Panoramic Laboratories, Electrochem Solutions, Active Implants Corporation, Micro Systems Engineering GmbH, Avail Medical Product, Mdmi Technologies Inc., Integer Holdings Corporation, Charles River Laboratories International, Inc, Boston Analytical, and Exova Group PLC. |
| Segments Covered | By End-user, By Device type, By Therapeutic area, By Services and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Global Medical Device Analytical Testing Outsourcing Market: Regional analysis
Asia Pacific accounts for the largest share in the global medical device analytical testing outsourcing market due to improving healthcare infrastructure and supportive government initiatives. Moreover, the rapid economic developments in the countries like China and India are likely to boost the regional market growth significantly during the forecast period.
North America is also expected to grow significantly during the forecast period due to the rapid growth in the manufacture of medical devices to fulfill the surging demand for efficient healthcare facilities in the region. The region is known as the top hub for the manufacturing of high-end, complex, and highly reliable medical devices.
Global Medical Device Analytical Testing Outsourcing Market: Competitive Players
Some of the significant players in the global medical device analytical testing outsourcing market are:
- Surpass
- BDC Laboratories
- NSF International
- Source Bioscience
- Medical Device Testing Services
- Charles River Laboratories
- Toxikon
- Wuxi Apptec
- Pace Analytical Services
- North American Science Associates
- Ministry SA
- Gateway Analytical
- Avomeen Analytical Services
- Envigo
- Elements Material Technology
- ASTM
- UL
- TÜV Rheinland
- BSI
- DEKRA
- TÜV SÜD
- Intertek
- Bureau Veritas
- Eurofins Scientific
- SGS
- Pharmaceutical Product Development, LLC
- Intertek Group PLC
- Pace Analytical Services, LLC
- West Pharmaceutical Services, Inc.
- Panoramic Laboratories
- Electrochem Solutions
- Active Implants Corporation
- Micro Systems Engineering GmbH
- Avail Medical Product
- Mdmi Technologies Inc.
- Integer Holdings Corporation
- Charles River Laboratories International, Inc
- Boston Analytical
- Exova Group PLC.
The Global Medical Device Analytical Testing Outsourcing Market is segmented as follows:
By End-use
- hospitals
- and others
By Device type
- reprocessed devices
- and others
By Therapeutic area
- diabetes care
- dental
- endoscopy
- drug delivery
- general & plastic surgery
- ophthalmic
- IVD
- orthopedic
- diagnostic imaging
- cardiology testing
- and others
By Services
- sterility testing
- bioburden testing
- physical testing
- material characterization
- extractable & leachable
Global Medical Device Analytical Testing Outsourcing Market: Regional Segment Analysis
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
What Reports Provides
- Full in-depth analysis of the parent market
- Important changes in market dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional markets
- Testimonials to companies in order to fortify their foothold in the market.
Table Of Content
FrequentlyAsked Questions
The growing prevalence of chronic diseases across the world is fueling the demand for medical device analytical testing outsourcing. Increasing stringent rules from the government regarding the quality certificate is propelling the growth of the global medical device analytical testing outsourcing market. Class II devices need premarket approvals & 510 (k) clearance, however, getting such clearance is a bit complex process and hence such emerging scenarios have boosted the growth of the global medical device analytical testing outsourcing market.
Some of the significant players in the global medical device analytical testing outsourcing market are Surpass, BDC Laboratories, NSF International, Source Bioscience, Medical Device Testing Services, Charles River Laboratories, Toxikon, Wuxi Apptec, Pace Analytical Services, North American Science Associates, Ministry SA, Gateway Analytical, Avomeen Analytical Services, Envigo, Elements Material Technology, ASTM, UL, TÜV Rheinland, BSI, DEKRA, TÜV SÜD, Intertek, Bureau Veritas, Eurofins Scientific, SGS, Pharmaceutical Product Development, LLC, Intertek Group PLC, Pace Analytical Services, LLC, and West Pharmaceutical Services, Inc.
Asia Pacific accounts for the largest share in the global medical device analytical testing outsourcing market due to improving healthcare infrastructure and supportive government initiatives. Moreover, the rapid economic developments in the countries like China and India are likely to boost the regional market growth significantly during the forecast period.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed