Medical Device Complaint Management Market Size, Share, Trends, Growth 2030

Medical Device Complaint Management Market By Service Type (Complaints Log/Intake, Product Surveillance & Regulatory Compliance, Returned/ Non-returned Product Analysis, and Resolve & Closure), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2023 - 2030
| Market Size in 2022 | Market Forecast in 2030 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 6.9 Billion | USD 12.4 Billion | 7.5% | 2022 |
Medical Device Complaint Management Industry Prospective:
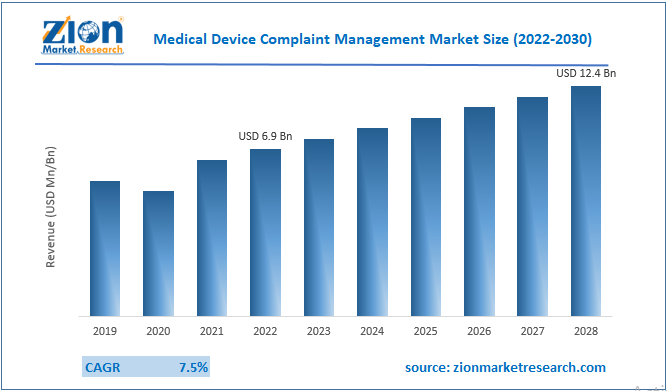
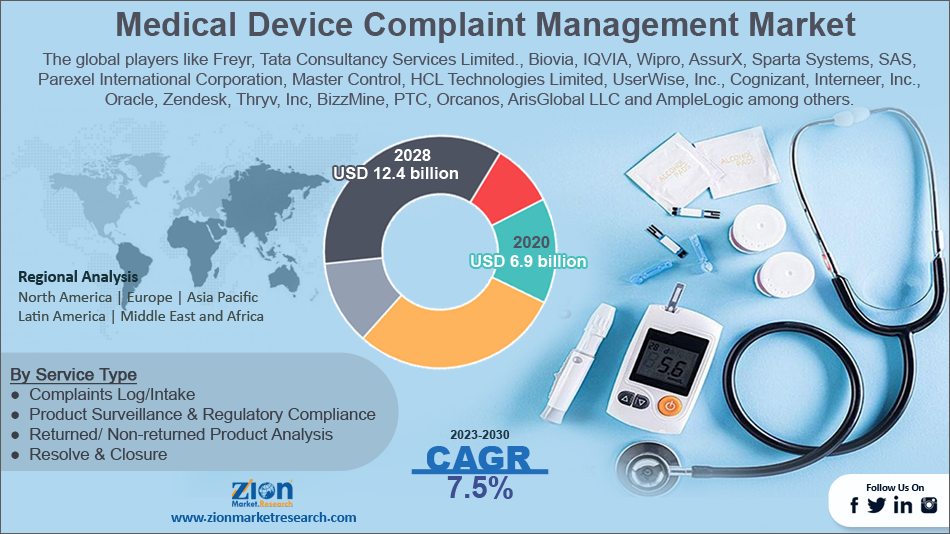
The global medical device complaint management market size was worth around USD 6.9 Billion in 2022 and is predicted to grow to around USD 12.4 Billion by 2030 with a compound annual growth rate (CAGR) of roughly 7.5% between 2023 and 2030.
The report analyzes the global medical device complaint management market’s drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the medical device complaint management industry.
Medical Device Complaint Management Market: Overview
Within a quality management system (QMS), complaint management software plays a crucial role in enabling manufacturers to promptly process concerns and, where necessary, involve suppliers. To support continuous improvement, complaint management software also offers feedback on product-in-use data to the design, planning, and process engineering teams of the manufacturer and/or supplier. The manufacturer's complaint gathering, analysis, and evaluation processes are made possible by the usage of a QMS concern and complaint management (CCM) software module. The evaluations that follow and vulnerability studies are crucial to the advancement of products and procedures. Software for managing complaints and concerns also enables manufacturers to streamline and synchronize supply chain operations with their integrated processes. Continuous improvement is made possible by CCM software, which includes internal problem-solving procedures as well as communication between companies and suppliers.
Key Insights
- As per the analysis shared by our research analyst, the global medical device complaint management market is estimated to grow annually at a CAGR of around 7.5% over the forecast period (2023-2030).
- In terms of revenue, the global medical device complaint management market size was valued at around USD 6.9 billion in 2022 and is projected to reach USD 12.4 billion, by 2030.
- The growing number of product recalls of medical devices is expected to drive market growth over the forecast period.
- Based on the service type, product surveillance & regulatory compliance are expected to dominate the market over the forecast period.
- Based on region, North America is expected to dominate the market during the forecast period.
Medical Device Complaint Management Market: Growth Drivers
The growing number of safety incidents associated with the use of medical devices drives the market growth
The number of medical device reports received by the US Food and Drug Administration (FDA) increased significantly between 2017 and 2019, according to the agency. When compared to 2017, when the FDA received 1,225,110 medical devices, the number received in 2019 was roughly 1,463,210, a 19.6% increase. The necessity to record any events involving the use of medical equipment and the growing awareness of patient safety are both responsible for this increase. This, in turn, is anticipated to boost the global medical device complaint management market demand.
Medical Device Complaint Management Market: Restraints
Improper classification of medical device complaints impedes the market growth
An endless cycle of escalating issues might result from improperly categorizing complaints. It distorts trending, tracking, and w. Regulators specifically check for it (and frequently issue observations for it) since it may lead to MDRs not being filed when they should be. In addition to causing backlogs, improper classification can make it more difficult to clear them by requiring the time-consuming process of re-opening and re-investigating closed files.
Medical Device Complaint Management Market: Opportunities
Innovative product launch by the key player provides a lucrative opportunity
The medical device complaint management market's key players are always working to develop novel product development strategies to obtain a competitive edge and meet the market's increasing demand. For instance, major firms are creating cutting-edge mobile application solutions to improve the management of client complaints. For instance, Intellect provides a platform for integrated mobile applications that users can use to file complaints, escalate issues, and create queries. The Intellect mobile app is conveniently offered on the App Store and Google Play Store, where it may be downloaded as an Intellect app or a mobile app with a different brand.
Medical Device Complaint Management Market: Challenges
Inadequate training for risk assignment and ranking poses a major challenge
The severity of each complaint should be rated, then it should be prioritized. However, a lot of complaint handlers lack the training necessary to do the amount of analysis needed for this job. A product engineer with extensive knowledge of both how to utilize the software that assigns risk numbers and the factors the program is considering to arrive at its response is often needed to assign risk effectively and consistently. Thus, inadequate training for risk assignment and ranking is expected to pose a major challenge to the medical device complaint management market growth over the forecast period.
Medical Device Complaint Management Market: Segmentation
The global medical device complaint management industry is segmented based on service type and region.
Based on the service type, the global market is bifurcated into complaints log/intake, product surveillance & regulatory compliance, returned/ non-returned product analysis, and resolve & closure. Product surveillance & regulatory compliance is expected to dominate the market over the forecast period. Because adverse events, product flaws, and medical device failures cause pain, injury, or even death, regulatory bodies, and consumers are becoming less tolerant. Furthermore, to properly track product performance and compliance, regulatory bodies continually emphasize the value of product surveillance.
The medical device vigilance/medical device reporting segment is anticipated to experience the fastest growth rate during the next years within the product surveillance segment. Regulations reforms carried out by regulatory bodies like the U.S. FDA are responsible for this lucrative expansion. For instance, the U.S. FDA has mandated that businesses submit to it immediately any concerns about adverse events, product defects, or failures relating to medical devices. These required regulatory improvements will come with severe penalties for non-compliance.
On the other hand, the complaints log/intake is expected to grow significantly during the forecast period. This section is a gateway mechanism for preventative or corrective action as well as post-market actions. It is the initial phase of complaint handling. Since the complaint must be evaluated to see if it qualifies as a reportable adverse event, complaint files are connected to the medical device reporting (MDR) event file. Additionally, it is required that medical device manufacturers record and monitor complaints by the quality system regulation (QSR), which is outlined in 21 CFR Part 820. Thus, this is expected to drive segment growth over the forecast period.
Recent Developments:
- In June 2021, InfectoPharm Arzneimittel und Consilium GmbH, the top pediatric pharmaceutical manufacturer in Germany, has chosen TrackWise Digital® as its end-to-end, next-generation quality management platform, according to a news release from Sparta Systems, a Honeywell company. To seamlessly integrate quality processes and data across its manufacturing operations and suppliers, InfectoPharm will implement TrackWise Digital's solution suite, which includes core QMS processes in addition to Complaints Handling, Supplier Quality Management, Document Management, and Training Management.
- In November 2021, a new module of IQVIA's range of Orchestrated Customer Engagement (OCE) products, Grants, and funding administration, has been made available. This initiative is anticipated to give the life sciences sector a way to effectively administer and supervise their international strategic giving programs.
Medical Device Complaint Management Market: Report Scope:
| Report Attributes | Report Details |
|---|---|
| Report Name | Medical Device Complaint Management Market Research Report |
| Market Size in 2022 | USD 6.9 Billion |
| Market Forecast in 2030 | USD 12.4 Billion |
| Growth Rate | CAGR of 7.5% |
| Number of Pages | 211 |
| Key Companies Covered | Freyr, Tata Consultancy Services Limited., Biovia, IQVIA, Wipro, AssurX, Sparta Systems, SAS, Parexel International Corporation, Master Control, HCL Technologies Limited, UserWise, Inc., Cognizant, Interneer, Inc., Oracle, Zendesk, Thryv, Inc, BizzMine, PTC, Orcanos, ArisGlobal LLC and AmpleLogic among others. |
| Segments Covered | By Service Type, AndBy Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2022 |
| Historical Year | 2017 to 2021 |
| Forecast Year | 2023 - 2030 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Medical Device Complaint Management Market: Regional Analysis
North America is expected to dominate the market during the forecast period
North America is expected to dominate the global medical device complaint management market over the forecast period. The growth in the region is attributed to the existence of strict regulatory standards for handling complaints. For instance, manufacturers, importers/exporters, and user facilities are all required by U.S. MDR regulatory guidelines to report adverse events and product failures relating to medical devices to the U.S. FDA on a mandatory basis. Moreover, the presence of a large number of medical device manufacturers and the growing number of product recalls is expected to drive market expansion in the region. For instance, as of November 2022, the FDA had announced 59 drug recalls and 55 medical device recalls. This contains numerous CPAP machines and CPAP masks made by Philips.
Besides, the Asia Pacific is expected to grow at the highest CAGR over the forecast period. The market in Asia Pacific is anticipated to grow due to the presence of notable multinational corporations like Wipro and Tata Consultancy Services (TCS) leading the complaint management market in the region. However, the European market is also growing at a significant rate during the projected period. The strict regulations and obligatory reporting requirements for medical device manufacturers to the European regulatory authorities of adverse occurrences and related safety steps taken are expected to cause the European market to grow at a considerable CAGR over the projected period. Manufacturers and businesses that don't follow these statutory rules risk severe fines. Thus, the aforementioned facts and figures support the market expansion in the region.
Medical Device Complaint Management Market: Competitive Analysis
The global medical device complaint management market is dominated by players like:
- Freyr
- Tata Consultancy Services Limited.
- Biovia
- IQVIA
- Wipro
- AssurX
- Sparta Systems
- SAS
- Parexel International Corporation
- Master Control
- HCL Technologies Limited
- UserWise Inc.
- Cognizant
- Interneer Inc.
- Oracle
- Zendesk
- Thryv Inc
- BizzMine
- PTC
- Orcanos
- ArisGlobal LLC
- AmpleLogic
- Among Others.
The global medical device complaint management market is segmented as follows:
By Service Type
- Complaints Log/Intake
- Product Surveillance & Regulatory Compliance
- Returned/ Non-returned Product Analysis
- Resolve & Closure
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Incorporating software helps organizations in maintaining better documentation. A multi-page electronic form ensures precise recording of complaint data along with reorganizing documentation into a simple process. This helps in maintaining transparency and improves decision-making related to customer complaints. The demand and popularity of the medical device complaint management market across the globe can be attributed to the growing software used to support the overall process of complaint management.
The major growth driver of the global medical device complaint management market in the future is the growing software used to support the overall process of complaint management. The shift of complaint tracking from paper to a digital system is anticipated to contribute notably toward the expansion of the medical device complaint management market globally over the estimated timeframe.
According to the report, the global market size was worth around USD 6.9 billion in 2022 and is predicted to grow to around USD 12.4 billion by 2030.
The global medical device complaint management market is expected to grow at a CAGR of 7.5% during the forecast period.
The global medical device complaint management market growth is expected to be driven by North America. It is currently the world’s highest revenue-generating market because there are strict regulatory standards for complaint management.
The global medical device complaint management market is dominated by players like Freyr, Tata Consultancy Services Limited., Biovia, IQVIA, Wipro, AssurX, Sparta Systems, SAS, Parexel International Corporation, Master Control, HCL Technologies Limited, UserWise, Inc., Cognizant, Interneer, Inc., Oracle, Zendesk, Thryv, Inc, BizzMine, PTC, Orcanos, ArisGlobal LLC and AmpleLogic among others.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed