Medical Device Testing Market Size, Share, Analysis, Trends, Growth, 2032

Medical Device Testing Market - By Device Class (Class I, Class II, and Class III), By Technology (Active Implant Medical Device, Active Medical Device, Non-Active Medical Device, In-Vitro Diagnostic Medical Device, Ophthalmic Medical Device, Orthopedic and Dental Medical Device, and Vascular Medical Device), And By Region- Global Industry Perspective, Comprehensive Analysis, and Forecast, 2024 - 2032-
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 9.17 Billion | USD 23.57 Billion | 9.9% | 2023 |
Medical Device Testing Market Insights
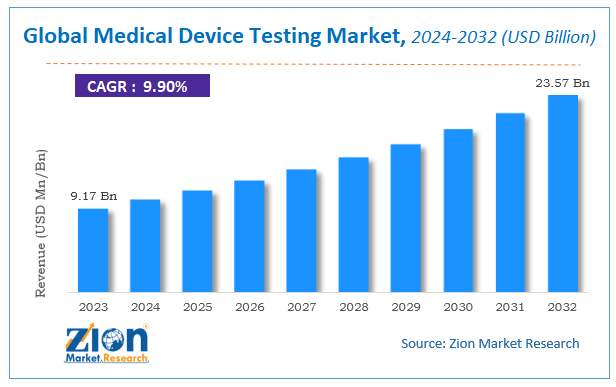
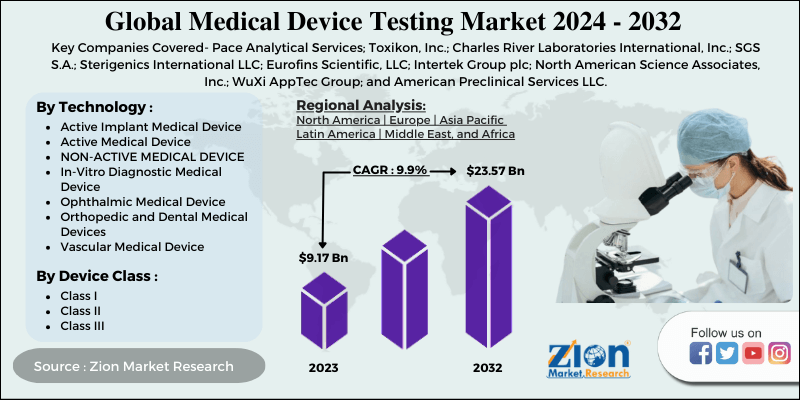
Zion Market Research has published a report on the global Medical Device Testing Market, estimating its value at USD 9.17 Billion in 2023, with projections indicating that it will reach USD 23.57 Billion by 2032. The market is expected to expand at a compound annual growth rate (CAGR) of 9.9% over the forecast period 2024-2032. The report explores the factors fueling market growth, the hitches that could hamper this expansion, and the opportunities that may arise in the Medical Device Testing Market industry. Additionally, it offers a detailed analysis of how these elements will affect market demand dynamics and market performance throughout the forecast period.
The report offers an assessment and analysis of the Medical Device Testing market on a global and regional level. The study offers a comprehensive assessment of the market competition, constraints, revenue estimates, opportunities, evolving trends, and industry-validated data. The report provides historical data from 2018 to 2022 along with a forecast from 2024 to 2032 based on revenue (USD Billion).
To know more about this report, Request A Sample Copy.
Medical Device Testing Market: Overview
Breakthroughs in the production of new testing methods for sterilizing and determining cytotoxicity are likely to present new growth opportunities for the medical device testing industry in the foreseeable future. Each medical equipment is assigned a protocol of testing requirements that are imposed by regulatory authorities including the U.S. FDA as well as the Chinese FDA to check the efficiency as well as determine the defects in medical equipment. The rise in then COVID cases across the globe has led to increasing in the testing of medical devices and this will enlarge the scope of the medical device testing industry over the forecast period. Massive outsourcing of medical equipment is set to steer the expansion of the medical device testing business over the forthcoming years.
Medical Device Testing Market: Growth Drivers
The onset of AI & Big Data analytics as well as IoT technologies has resulted in massive demand for adding new features in medical devices to improve disease identification at the initial phase, thereby resulting in a rise in the market demand. Apart from this, the necessity for treating the disease at an early stage along with a need for improving diagnosis, medical or drug treatment, and patient monitoring will drive the industry trends. The thriving healthcare sector will prompt the demand for medical device testing activities in the foreseeable future. This, in turn, will embellish the growth of the medical device testing industry over the assessment timeframe.
Furthermore, the surge in the number of product recalls as well as the escalating necessity of high-quality medical devices will prompt the expansion of the medical device testing industry over the assessment period. With spinal cord stimulators assisting patients suffering from pain, the market for medical device testing is likely to expand by leaps & bounds over 2024 – 2032.
Medical Device Testing Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Medical Device Testing Market |
| Market Size in 2023 | USD 9.17 Billion |
| Market Forecast in 2032 | USD 23.57 Billion |
| Growth Rate | CAGR of 9.9% |
| Number of Pages | 150 |
| Key Companies Covered | Pace Analytical Services; Toxikon, Inc.; Charles River Laboratories International, Inc.; SGS S.A.; Sterigenics International LLC; Eurofins Scientific, LLC; Intertek Group plc; North American Science Associates, Inc.; WuXi AppTec Group; and American Preclinical Services LLC. |
| Segments Covered | By Technology, By Device Class, And By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Medical Device Testing Market: Regional Insights
North American Market To Register Exponential Growth Over 2024 – 2032
The expansion of the medical device testing industry in North America over the forecast period is owing to strict laws by the regulatory bodies in the U.S. and Canada for maintaining safety & quality standards. A rise in the requirement for TIC services in the sub-continent will further upsurge the regional market growth. Apart from this, the rise in product design complexity and the need for reducing the costs of the product will result in humungous market penetration in the region within the next couple of years. The thriving pharma sector coupled with the large-scale presence of mammoth medical device manufacturing firms in the sub-continent will manifest in humungous growth of the medical device testing industry in the region over the anticipated period.
Key Market Players & Competitive Landscape
Key players profiled in the report include
- Pace Analytical Services
- Toxikon, Inc.
- Charles River Laboratories International, Inc.
- SGS S.A.
- Sterigenics International LLC
- Eurofins Scientific, LLC
- Intertek Group plc
- North American Science Associates, Inc.
- WuXi AppTec Group
- American Preclinical Services LLC.
The global Medical Device Testing Market is segmented as follows:
By Technology
- Active Implant Medical Device
- Active Medical Device
- NON-ACTIVE MEDICAL DEVICE
- In-Vitro Diagnostic Medical Device
- Ophthalmic Medical Device
- Orthopedic and Dental Medical Devices
- Vascular Medical Device
By Device Class
- Class I
- Class II
- Class III
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
The onset of AI & Big Data analytics as well as IoT technologies has resulted in massive demand for adding new features in medical devices to improve the disease identification at initial phase, thereby resulting in rise in the market demand. Apart from this, necessity for treating disease at early stage along with need for improving diagnosis, medical or drug treatment, and patient monitoring will drive the industry trends.
According to Zion market research report, the global Medical Device Testing Market, estimating its value at USD 9.17 Billion in 2023, with projections indicating that it will reach USD 23.57 Billion by 2032. The market is expected to expand at a compound annual growth rate (CAGR) of 9.9% over the forecast period 2024-2032.
North America will contribute lucratively towards the global market size over the estimated timeline. The regional market surge is due to strict laws by the regulatory bodies in the U.S. and Canada for maintaining safety & quality standards. Rise in the requirement of TIC services in the sub-continent will further upsurge the regional market growth.
The key market participants include Pace Analytical Services; Toxikon, Inc.; Charles River Laboratories International, Inc.; SGS S.A.; Sterigenics International LLC; Eurofins Scientific, LLC; Intertek Group plc; North American Science Associates, Inc.; WuXi AppTec Group; and American Preclinical Services LLC.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed