Medical Inhaled Nitric Oxide Market Size, Share, Analysis, Trends, Growth Report, 2030

Medical Inhaled Nitric Oxide Market By Product Type (Inhaled Nitric Oxide Delivery Systems, Nitric Oxide Sensors, and Monitors), By Route of Administration (Inhaled Nitric Oxide Gas and Inhaled Nitric Oxide with Oxygen Mixtures), By Application (Cardiovascular Disorders and Respiratory Disorders), By End User (Hospitals, Clinics, and Other End users), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2023 - 2030
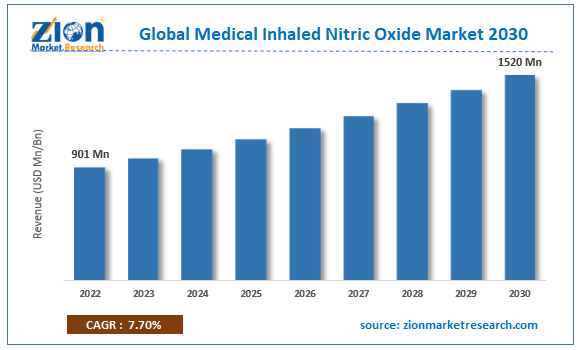
| Market Size in 2022 | Market Forecast in 2030 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 901 Million | USD 1,520 Million | 7.7% | 2022 |
Medical Inhaled Nitric Oxide Industry Prospective:
The global medical inhaled nitric oxide market size was worth around USD 901 million in 2022 and is predicted to grow to around USD 1,520 million by 2030 with a compound annual growth rate (CAGR) of roughly 7.7% between 2023 and 2030.
Medical Inhaled Nitric Oxide Market: Overview
Medical inhaled nitric oxide is a specialized treatment within the pharmaceutical and medical sector, designed to address respiratory and cardiovascular issues. This therapeutic gas, composed of nitric oxide, is administered through inhalation to patients in controlled medical environments. It plays a crucial role in improving oxygenation and reducing pulmonary hypertension by selectively dilating pulmonary blood vessels. Medical inhaled nitric oxide is employed in various scenarios, including the treatment of pulmonary arterial hypertension (PAH), enhancing oxygenation in neonates with hypoxic respiratory failure, and assisting in managing adult respiratory distress syndrome (ARDS). Due to its precise administration requirements, this treatment is primarily utilized in hospitals, contributing significantly to advanced patient care and well-being.
Key Insights
- As per the analysis shared by our research analyst, the global medical inhaled nitric oxide industry is estimated to grow annually at a CAGR of around 7.7% over the forecast period (2023-2030).
- In terms of revenue, the global medical inhaled nitric oxide market size was valued at around USD 901 million in 2022 and is projected to reach USD 1,520 million, by 2030.
- The global medical inhaled nitric oxide market is projected to grow at a significant rate due to the increasing prevalence of pulmonary arterial hypertension (PAH) and related respiratory disorders.
- Based on product type segmentation, the nitric oxide delivery systems segment was predicted to hold maximum market share in the year 2022.
- Based on route of administration segmentation, inhaled nitric oxide gas was the leading revenue generator in 2022.
- Based on application segmentation, respiratory disorders was the leading revenue generator in 2022.
- Based on end-user segmentation, hospitals was the leading revenue generator in 2022.
- On the basis of region, North America was the leading revenue generator in 2022.
 Request Free Sample
Request Free Sample
Medical Inhaled Nitric Oxide Market: Growth Drivers
Increasing prevalence of pulmonary arterial hypertension (PAH) and related respiratory disorders is expected to boost market growth during the forecast period.
The escalating prevalence of pulmonary arterial hypertension (PAH) and associated respiratory disorders has emerged as a prominent driver propelling the growth of the medical inhaled nitric oxide market. PAH is a debilitating condition characterized by elevated blood pressure in the pulmonary arteries, causing strain on the heart and restricting blood flow to the lungs. This results in symptoms such as shortness of breath, fatigue, and chest pain.
PAH can be idiopathic or associated with underlying conditions like connective tissue diseases, congenital heart defects, and liver disease. Recent statistics underscore the pressing need for effective PAH treatments. According to the Pulmonary Hypertension Association, PAH affects about 15 to 50 individuals per one million adults globally. Furthermore, PAH disproportionately impacts women and tends to manifest between the ages of 30 and 60. The growing awareness and diagnosis of PAH, coupled with advancements in medical treatment options, have spurred the demand for therapies like inhaled nitric oxide that can offer targeted vasodilation of pulmonary blood vessels. As a result, the medical inhaled nitric oxide market is experiencing substantial growth as healthcare providers seek innovative solutions to address the rising burden of PAH and its related respiratory complications.
Medical Inhaled Nitric Oxide Market: Restraints
The complexity and cost associated with its administration and delivery systems is likely to hamper market expansion
The medical inhaled nitric oxide industry faces a significant restraint in the form of the complexity and cost associated with its administration and delivery systems. Inhaled nitric oxide therapy requires specialized equipment and infrastructure to accurately deliver the gas in controlled concentrations to patients. This involves the use of intricate delivery systems, including monitors to regulate the amount of nitric oxide administered. The technical complexity of these systems demands specialized training for healthcare professionals, adding a layer of complexity to its adoption. Moreover, the cost of implementing and maintaining these sophisticated delivery systems can be substantial.
Alongside the equipment expenses, there are ongoing costs for maintenance, calibration, and the necessary gas supply. These factors collectively contribute to the financial burden on healthcare facilities, potentially limiting the widespread adoption of inhaled nitric oxide therapy. This is particularly relevant in healthcare systems where resource allocation is a critical consideration. According to a study published in the "American Journal of Respiratory and Critical Care Medicine," the cost of inhaled nitric oxide therapy for neonates with respiratory failure can be considerable, reaching an estimated USD 18,000 to USD 22,000 per patient per day. Additionally, the need for skilled personnel and the availability of specialized infrastructure further add to the operational costs.
Medical Inhaled Nitric Oxide Market: Opportunities
The ongoing research and development efforts aimed at improving delivery systems and reducing the complexity and costs to provide growth opportunities
The medical inhaled nitric oxide market is poised to seize a significant opportunity through ongoing research and development endeavors aimed at enhancing the delivery systems and streamlining the complexities and costs related to its administration. As the demand for targeted and efficient therapies grows, researchers and manufacturers are working diligently to refine the process of delivering inhaled nitric oxide to patients. These efforts encompass the development of user-friendly and efficient delivery devices, which could simplify the administration process, reduce training requirements, and potentially lower the financial burden on healthcare institutions.
Technological advancements play a pivotal role in this opportunity, as they enable the creation of innovative delivery systems that offer improved precision and ease of use. By focusing on refining dosages, treatment durations, and patient-specific requirements, ongoing research contributes to the optimization of inhaled nitric oxide therapy. Such advancements have the potential to revolutionize the treatment landscape, enhancing patient care and expanding the adoption of this valuable therapeutic approach.
Medical Inhaled Nitric Oxide Market: Challenges
The intricate regulatory landscape and the need for stringent compliance due to the gas's status as a controlled substance might challenge market growth
The medical inhaled nitric oxide industry faces a significant challenge in navigating the intricate regulatory landscape and adhering to stringent compliance requirements. This complexity arises primarily from the gas's status as a controlled substance due to its potential environmental and health impacts. Regulatory bodies often impose strict guidelines and oversight on the production, distribution, and use of inhaled nitric oxide to ensure its safe and responsible use. For instance, in the United States, inhaled nitric oxide is classified as a drug by the Food and Drug Administration (FDA) and requires approval for specific indications.
The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) designates nitric oxide as a hazardous substance, further underscoring the need for careful handling and disposal. Such stringent regulations extend to international markets as well, adding layers of complexity for manufacturers and healthcare providers operating across borders. Adhering to these regulations demands substantial resources and expertise to ensure that the gas is manufactured, stored, transported, and administered in a manner that safeguards patient health and minimizes environmental impact. The evolving nature of regulatory standards and the potential for legal consequences for non-compliance pose a substantial challenge to market participants. Overcoming this challenge requires robust collaboration between regulatory authorities, manufacturers, and healthcare facilities to strike a balance between patient care and environmental responsibility.
Medical Inhaled Nitric Oxide Market: Segmentation
The global medical inhaled nitric oxide market is segmented based on product type, route of administration, end-user, and region.
Based on product type, the global market segments are inhaled nitric oxide delivery systems, nitric oxide sensors, and monitors. At present, the global market is dominated by the nitric oxide delivery systems segment. These systems encompass the equipment and mechanisms necessary for the controlled administration of inhaled nitric oxide to patients. They play a crucial role in regulating the concentration of nitric oxide delivered and ensuring its safe and effective use.
Based on route of administration the global medical inhaled nitric oxide industry is categorized as inhaled nitric oxide gas, inhaled nitric oxide with oxygen mixtures. Out of these, inhaled nitric oxide gas was the largest shareholding segment in the global market. Inhaled nitric oxide gas typically leads in the medical inhaled nitric oxide market because it directly delivers pure nitric oxide for targeted therapeutic effects on pulmonary blood vessels, making it the primary mode of administration for desired outcomes.
Based on application the global medical inhaled nitric oxide market is categorized as cardiovascular disorders and respiratory disorders. Out of these, the respiratory disorders segment was the largest shareholding segment in the global market. Inhaled nitric oxide gas generally leads in the application of respiratory disorders within the medical inhaled nitric oxide market, as its mechanism of improving oxygenation and reducing pulmonary hypertension aligns well with addressing respiratory challenges.
Based on end-user, the global medical inhaled nitric oxide industry is categorized as hospitals and clinics. Out of these, hospital segment was the largest shareholding segment in the global market due to the specialized resources and infrastructure required for administering this therapy effectively.
Medical Inhaled Nitric Oxide Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Medical Inhaled Nitric Oxide Market |
| Market Size in 2022 | USD 901 Million |
| Market Forecast in 2030 | USD 1520 Million |
| Growth Rate | CAGR of 7.7% |
| Number of Pages | 214 |
| Key Companies Covered | Bellerophon Therapeutics, Praxair Technology Inc. (Linde plc), Mallinckrodt Pharmaceuticals, GE Healthcare, Mitsubishi Tanabe Pharma Corporation, Vero Biotech LLC, Messer Group, Air Liquide, Nu-Med Plus, Beyond Air Inc., and others. |
| Segments Covered | By Product Type, By Route of Administration, By Application, By End User, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2022 |
| Historical Year | 2017 to 2021 |
| Forecast Year | 2023 - 2030 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Medical Inhaled Nitric Oxide Market: Regional Analysis
North America to lead the market during the forecast period
North America, particularly the United States, stands out as a leading region in the global medical inhaled nitric oxide market. This prominence can be attributed to the region's advanced healthcare infrastructure, robust research & development capabilities, and a relatively high prevalence of conditions such as pulmonary arterial hypertension and respiratory distress syndrome that require specialized treatments like inhaled nitric oxide therapy. The United States, with its well-established healthcare system and extensive network of hospitals and medical centers, has been at the forefront of adopting cutting-edge medical technologies, contributing to its leadership position in the market.
The medical inhaled nitric oxide market in the United States is expected to grow at a CAGR of 6.8% from 2022 to 2030.
Medical Inhaled Nitric Oxide Market: Recent Developments
- In August 2022, Zylo Therapeutics Inc. received a two-year, USD 600,000 Small Business Innovation Research (SBIR) grant for developing the company's final formulation of its patented topical medication that releases nitric oxide to treat onychomycosis. The National Institute of Allergy and Infectious Diseases, a part of the National Institutes of Health (NIH), is funding the grant.
- In August 2022, SaNOtize Research & Development Corp. received an oversubscribed USD 24 million Series B fundraising to further its nitric oxide-based medicines pipeline. The company's nitric oxide nasal spray (NONS), which has been demonstrated in clinical tests to cure and prevent COVID-19 infection, is the principal therapeutic in development.
Medical Inhaled Nitric Oxide Market: Competitive Analysis
The global medical inhaled nitric oxide market is dominated by players like:
- Bellerophon Therapeutics
- Praxair Technology, Inc. (Linde plc)
- Mallinckrodt Pharmaceuticals
- GE Healthcare
- Mitsubishi Tanabe Pharma Corporation
- Vero Biotech LLC
- Messer Group
- Air Liquide
- Nu-Med Plus
- Beyond Air, Inc.
The global medical inhaled nitric oxide market is segmented as follows:
By Product Type
- Inhaled Nitric Oxide Delivery Systems
- Nitric Oxide Sensors and Monitors
By Route of Administration
- Inhaled Nitric Oxide Gas
- Inhaled Nitric Oxide with Oxygen Mixtures
By Application
- Cardiovascular Disorders
- Respiratory Disorders
By End User
- Hospitals
- Clinics
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Medical inhaled nitric oxide is a specialized treatment within the pharmaceutical and medical sector, designed to address respiratory and cardiovascular issues. This therapeutic gas, composed of nitric oxide, is administered through inhalation to patients in controlled medical environments.
The global medical inhaled nitric oxide market cap may grow owing to the Increasing prevalence of pulmonary arterial hypertension (PAH) and related respiratory disorders. Significant growth opportunities can be expected due to the ongoing research and development efforts aimed at improving delivery systems and reducing complexity and costs.
According to study, the global medical inhaled nitric oxide market size was worth around USD 901 million in 2022 and is predicted to grow to around USD 1,520 million by 2030.
The CAGR value of the medical inhaled nitric oxide market is expected to be around 7.7% during 2023-2030.
The global medical inhaled nitric oxide market growth is expected to be driven by North America. It is currently the world’s highest revenue-generating market owing to the higher prevalence rate, high spending, and adoption of the latest medical technologies.
The global medical inhaled nitric oxide market is led by players like Bellerophon Therapeutics, Praxair Technology, Inc. (Linde plc), Mallinckrodt Pharmaceuticals, GE Healthcare, Mitsubishi Tanabe Pharma Corporation, Vero Biotech LLC, Messer Group, Air Liquide, Nu-Med Plus, and Beyond Air, Inc.
The report analyzes the global medical inhaled nitric oxide market’s drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the medical inhaled nitric oxide industry.
Choose License Type
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed