MRD Testing Market Size, Share, Trends, Growth and Forecast 2032

MRD Testing Market By End-User (Academic & Research Institutes, Diagnostic Laboratories, and Hospitals & Speciality Clinics), By Application (Solid Tumors and Hematological Malignancy), By Technology (Next Generation Sequencing (NGS), Flow Cytometry, Polymerase Chain Reaction (PCR), and Others), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2024 - 2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 2,145.76 Million | USD 6,707.24 Million | 13.50% | 2023 |
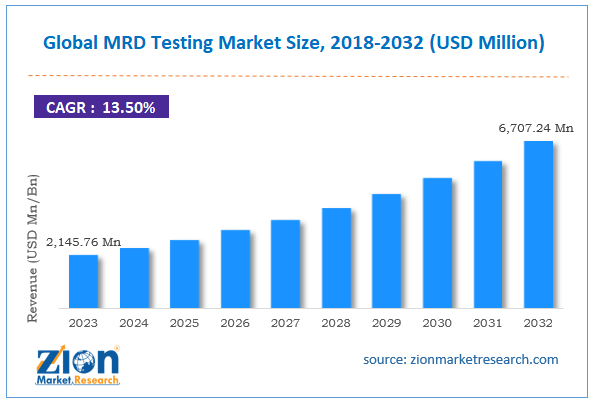
MRD Testing Industry Prospective:
The global MRD testing market size was worth around USD 2,145.76 million in 2023 and is predicted to grow to around USD 6,707.24 million by 2032 with a compound annual growth rate (CAGR) of roughly 13.50% between 2024 and 2032.
MRD Testing Market: Overview
MRD testing is also known as minimal or measurable residual disease testing. It is used for testing if the cancer treatments are showing desired results or not. MRD testing is also used for drafting further treatment plans for cancer patients. Minimal residual disease testing procedures cannot be used for all cancers at present. They are most effective in select cancer types such as myeloma, lymphoma, and leukemia. However, several ongoing research has currently been conducted globally to understand the application of the testing procedure for other cancer variants. MRD testing is considered in the personalized medicine segment since the testing process can help curate specific treatment plans best suited to the medical needs of the patient. MRD testing is extremely helpful in determining the efficiency of the cancer treatment plan. It is used for gaining information on how well the body is reacting to the treatment. As compared to other tests, minimal residual disease testing can be more effective in finding out if the patient is in remission. Furthermore, it also helps detect cases of cancer recurrence faster than other tests. MRD testing is a heavily; specialized form of cancer testing and can only be conducted at highly advanced facilities thus restricting the industry’s adoption rate. However, more efforts are being directed toward making the testing process more accessible among the patient groups.
Key Insights:
- As per the analysis shared by our research analyst, the global MRD testing market is estimated to grow annually at a CAGR of around 13.50% over the forecast period (2024-2032)
- In terms of revenue, the global MRD testing market size was valued at around USD 2,145.76 million in 2023 and is projected to reach USD 6,707.24 million, by 2032.
- The market is projected to grow at a significant rate due to the rising number of patients with cancer conditions
- Based on the end-user, the hospitals & specialty clinics segment is growing at a high rate and will continue to dominate the global market as per industry projection
- Based on the technology, the polymerase chain reaction (PCR) segment is anticipated to command the largest market share
- Based on region, North America is projected to dominate the global market during the forecast period
 Request Free Sample
Request Free Sample
MRD Testing Market: Growth Drivers
Rising number of patients with cancer conditions may drive the market growth rate
The global MRD testing market is expected to generate higher growth due to the increasing number of patients who are currently living with cancer. It is one of the world’s leading causes of medically-induced deaths. As per recent findings published by Our World in Data, around one in every six deaths globally is caused by cancer. There are several types of cancer which makes it difficult for healthcare experts and drug developers to draft a standard treatment program for curing the disease. As of present times, cancer does not have specific treatment plans and each process may depend on the disease stage and patient’s overall health conditions. Additionally, the treatment plan is also at the discretion of the medical professional handling the case. Leukemia, for instance, is a type of cancer in the blood. As per Cleveland Clinic, it is the 10th most common type of cancer in the US. Leukemia is caused by the mutation of deoxyribonucleic acid causing the leukemia cells to behave like white blood cells but abnormally. Leukemia is further classified into several types depending on speed and cell type. For instance, as per the former type, the primary classifications are chronic leukemia and acute leukemia. Based on cell type, the disease is classified into lymphocytic leukemia and myelogenous. As per the National Institutes of Health (NIH), more than 2.43 prevalent cases of leukemia exist across the globe. It mostly occurs in people between the ages of 65 to 74. The main cause of the disease currently remains unknown however, certain risk factors have been chalked out by the researchers. Smoking, previous cancer treatment, specific genetic disorders, exposure to industrial chemicals, and family history of leukemia are associated with the condition.
Higher consumer awareness about cancer and treatment plans may develop more demand for the novel testing method
As compared to the last decade, more people are aware of cancer, different types of disease, and the availability of medical treatment for managing the condition. The growing consumer awareness could benefit the global MRD testing market. In February 2023, the World Health Organization (WHO) launched the Global Breast Cancer Initiative Framework through which the agency aims to save over 2.5 million lives by 2040. In September 2023, the International Agency for Research on Cancer (IARC) announced the launch of the EASTER project. It is a new cervical cancer screening for validating technologies that can be used for screening and diagnosing the condition.
MRD Testing Market: Restraints
Limited application of the procedure at present times may restrict the market expansion rate
The global industry for MRD testing is expected to be restricted due to the limited application of the procedure across cancer types. At present, this method of testing cancer treatment efficiency is currently used only for a limited set of cancer variants. However, the disease has several forms that continue to take the lives of millions of people across the globe. Additionally, the lack of infrastructure supporting higher accessibility to MRD testing further limits the industry's expansion rate.
MRD Testing Market: Opportunities
Steady growth in investments toward comprehensive growth of MRD testing may generate new expansion possibilities
The global MRD testing market will generate extensive growth avenues during the forecast period. The industry is registering a surge in investments across processes related to MRD testing. For instance, investments can be recorded in making the testing process accessible to the common people. Additionally, players are focusing on improving the test’s efficiency rate by developing more accurate measurable residual disease testing. In April 2023, Corgenix, a company owned by the Sebia Group which is a leader in the diagnosis and monitoring of multiple myeloma, announced the launch of a novel MRD test based on serum. The new Laboratory Developed Test (LDT) is called M-inSight® and is licensed by Clinical Laboratory Improvement Amendments (CLIA). M-inSight is a targeted and personalized mass spectrometry assay and will be used for detecting monoclonal protein (M-protein) with unprecedented sensitivity. In February 2024, Myriad Genetics, Inc. announced a partnership with the Japan-based National Cancer Center Hospital East (NCCHE). The former is a leading player in precision medicine and genetic testing. The collaboration will work toward studying the predictive and prognostic value of MRD testing.
Ascending work toward making cancer-care available for the underprivileged segment of society will open new doors for growth
Cancer diagnosis, treatment, and management are expensive. Regional governments across the globe are working toward making cancer-care more affordable and accessible for the underprivileged segments of society. For instance, as per the first initiative Rays of Hope: Cancer Care for All by the International Atomic Energy Agency (IAEA), the flagship Anchor Center was inaugurated in Türkiye for Central Asia and Europe thus creating growth scope for the global MRD testing market. India, on the other hand, has recently launched the Ayushman Bharat Pradhan Mantri Jan Arogya Yojana (PMJAY) through which people below the poverty line can obtain a medium insurance of INR 5 lakhs per family annually and use it for all secondary and tertiary care conditions.
MRD Testing Market: Challenges
Concerns over the lack of sufficient medical reimbursements for MRD testing could challenge the expansion rate
The global industry for MRD testing is projected to be challenged by the lack of medical insurance policies supporting measurable residual disease testing. It is a sophisticated testing method and cannot be carried out at regular diagnostic centers. This means that the insurance companies may not cover MRD testing given the specialized status of the testing method making it difficult for a large part of the potential users to obtain the benefits of MRD testing and this challenges the industry’s growth rate.
MRD Testing Market: Segmentation
The global MRD testing market is segmented based on end-user, application, technology, and region.
Based on the end-user, the global market segments are academic & research institutes, diagnostic laboratories, and hospitals & specialty clinics. In 2023, the highest demand was observed in the hospitals & specialty clinics segment. Minimal residual disease testing is a complex procedure and requires specialized equipment. Additionally, it can only be carried out by trained experts. The relatively limited number of facilities offering these services are mostly associated with hospitals and specialty clinics as these units are equipped with state-of-the-art systems. The global hospital market was valued at over USD 2.29 trillion in 2023.
Based on application, the global MRD testing industry is segmented into solid tumors and hematological malignancies.
Based on the technology, the global market segments are next-generation sequencing (NGS), flow cytometry, polymerase chain reaction (PCR), and others. In 2023, the highest growth was registered in the polymerase chain reaction (PCR) segment. The technology has been mastered over the years and hence more facilities offer PCR-based MRD testing. The NGS segment is growing at a steady pace. MRD testing has an average success rate of more than 70%.
MRD Testing Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | MRD Testing Market |
| Market Size in 2023 | USD 2,145.76 Million |
| Market Forecast in 2032 | USD 6,707.24 Million |
| Growth Rate | CAGR of 13.50% |
| Number of Pages | 217 |
| Key Companies Covered | Sysmex Corporation, Bio-Techne Corporation, Adaptive Biotechnologies Corporation, Foundation Medicine Inc. (a member of the Roche Group), NeoGenomics Laboratories Inc., Guardant Health Inc., Invivoscribe Inc., F. Hoffmann-La Roche Ltd., Illumina Inc., Thermo Fisher Scientific Inc., Genomic Health (acquired by Exact Sciences Corporation), Natera Inc., ArcherDX (now part of Invitae Corporation), Bio-Rad Laboratories Inc., Qiagen N.V., and others. |
| Segments Covered | By End-User, By Application, By Technology, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
MRD Testing Market: Regional Analysis
North America is expected to deliver the optimum results in the coming years
The global MRD testing market will be dominated by North America during the projection period. In 2023, North America was the leading market and contributed to over 44.9% of the total market share. The increasing number of cancer patients along with the existence of a robust cancer diagnostic and treatment healthcare infrastructure helps the US lead the regional market growth rate. The country has several dedicated cancer research facilities that are equipped to develop novel methods of cancer diagnosis and treatment. Additionally, cancer funding in the US tops other countries across the globe. In September 2023, the US government allocated USD 330 million for the Cancer Moonshot Initiative. The funds will also be used for improvements in healthcare research and treatment. In January 2024, US-based Tempus announced the launch of xM MRD (formerly xM). The company specializes in precision medicine and artificial intelligence (AI). The new tool will be used for assessing MRD in patients with colorectal cancer (CRC). However, it is currently available for research purposes only. In March 2024, Personalis, Inc. presented data related to the NeXT Personal® tumor-informed whole genome-based MRD testing at the annual meeting of the American Association for Cancer Research (AACR).
MRD Testing Market: Competitive Analysis
The global MRD testing market is led by players like:
- Sysmex Corporation
- Bio-Techne Corporation
- Adaptive Biotechnologies Corporation
- Foundation Medicine Inc. (a member of the Roche Group)
- NeoGenomics Laboratories Inc.
- Guardant Health Inc.
- Invivoscribe Inc.
- F. Hoffmann-La Roche Ltd.
- Illumina Inc.
- Thermo Fisher Scientific Inc.
- Genomic Health (acquired by Exact Sciences Corporation)
- Natera Inc.
- ArcherDX (now part of Invitae Corporation)
- Bio-Rad Laboratories Inc.
- Qiagen N.V.
The global MRD testing market is segmented as follows:
By End-User
- Academic & Research Institutes
- Diagnostic Laboratories
- Hospitals & Speciality Clinics
By Application
- Solid Tumors
- Hematological Malignancy
By Technology
- Next Generation Sequencing (NGS)
- Flow Cytometry
- Polymerase Chain Reaction (PCR)
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
MRD testing is also known as minimal or measurable residual disease testing.
The global MRD testing market is expected to generate higher growth due to the increasing number of patients who are currently living with cancer.
According to study, the global MRD testing market size was worth around USD 2,145.76 million in 2023 and is predicted to grow to around USD 6,707.24 million by 2032.
The CAGR value of MRD testing market is expected to be around 13.50% during 2024-2032.
The global MRD testing market will be dominated by North America during the projection period.
The global MRD testing market is led by players like Sysmex Corporation, Bio-Techne Corporation, Adaptive Biotechnologies Corporation, Foundation Medicine, Inc. (a member of the Roche Group), NeoGenomics Laboratories, Inc., Guardant Health, Inc., Invivoscribe, Inc., F. Hoffmann-La Roche Ltd., Illumina, Inc., Thermo Fisher Scientific Inc., Genomic Health (acquired by Exact Sciences Corporation), Natera, Inc., ArcherDX (now part of Invitae Corporation), Bio-Rad Laboratories, Inc. and Qiagen N.V.
The report explores crucial aspects of the MRD testing market including detailed discussion of existing growth factors and restraints while also browsing future growth opportunities and challenges that impact the market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed