Oncology Biosimilar Market Size, Share, Growth Report 2032

Oncology Biosimilar Market By Cancer Type (Breast Cancer, Colorectal Cancer, Blood Cancer, Neutropenia Cancer, Non-Small Cell Lung Cancer, And Others), By Drug Type (MAb, Immunomodulators, Hematopoietic Agents, G-CSF, And Others), By Distribution Channel (Retail Pharmacies, Hospital Pharmacy, And Online Pharmacy), And By Region - Global And Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, And Forecasts 2024-2032
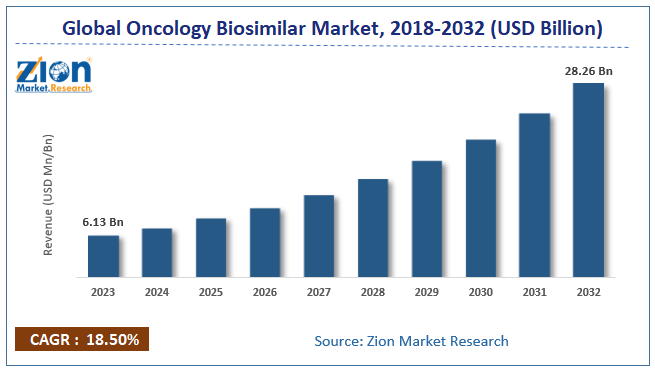
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 6.13 Billion | USD 28.26 Billion | 18.50% | 2023 |
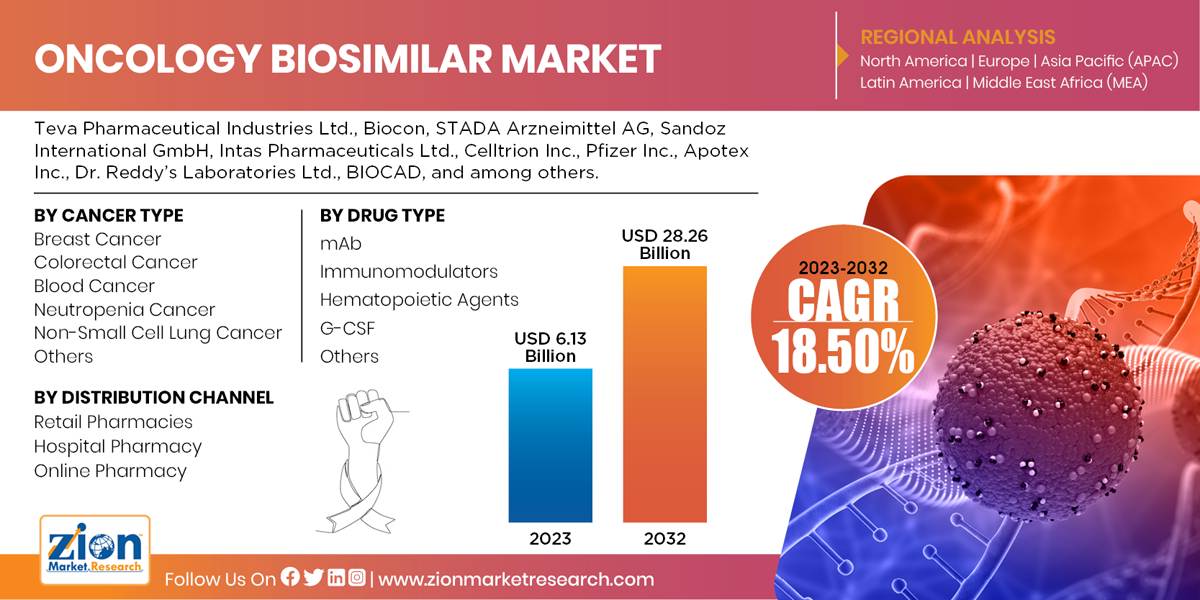
The global oncology biosimilar market size was worth around USD 6.13 billion in 2023 and is predicted to grow to around USD 28.26 billion by 2032 with a compound annual growth rate (CAGR) of roughly 18.50% between 2024 and 2032.
The report covers forecast and analysis for the oncology biosimilar market on a global and regional level. The study provides historical data from 2018 to 2022 along with a forecast from 2024 to 2032 based revenue (USD Billion).
Oncology Biosimilar Market: Overview
Biosimilar is a biological medical product which is an identical copy of the original product manufactured by the companies. Biosimilar is the approved version of innovative products and can only be manufactured after the patent expiry of the original product. Oncology biosimilar is different as compared to the biologics in terms of complexity and manufacturing process. The biosimilar is much cheaper as compared to branded and approved products which are available in the market. There are several biologics available in the market for oncology which is on its way of patent expiry. Patent expiry of branded biologics will increase the biosimilar market. Biosimilar can be used for the treatment of various types of cancer such as lung cancer, kidney cancer, and others which are expected to open the gates of opportunities for the biosimilar market in the near future.
Key Insights
- As per the analysis shared by our research analyst, the global oncology biosimilar market is estimated to grow annually at a CAGR of around 18.50% over the forecast period (2024-2032).
- In terms of revenue, the global oncology biosimilar market size was valued at around USD 6.13 billion in 2023 and is projected to reach USD 28.26 billion, by 2032.
- Based on drug type, the G-CSF segment is expected to grow at the fastest rate during the forecast period
- Based on the distribution channel, the hospital pharmacy segment is expected to dominate the market over the forecast period.
- Based on region, North America is expected to hold a prominent market share over the forecast period.
Oncology Biosimilar Market: Growth Drivers
One of the major factors driving the oncology biosimilar market is the increasing demand for affordable therapies due to the constant increase in cancer patients worldwide. Various pharmaceutical companies are involved in the development of oncology biosimilar and generic medicines for the treatment of cancer at all stages and early detection of cancer. Due to the high demand for the cancer treatment, there is a rapid rate of approval observed in past few years which are expected to drive the oncology biosimilar market. The first biosimilar was approved in 2006 in Europe, and in the U.S. first biosimilar was approved in 2015 after the approval of first biosimilar FDA has approved five biosimilars in 2017. There is a number of applications pending for the approval of oncology biosimilar and their approval in future are expected to support the growth of oncology biosimilar market.
Oncology Biosimilar Market: Segmentation
The study provides a decisive view on the oncology biosimilar market by segmenting the market based on cancer type, drug type, distribution channel, and regions. All the segments have been analyzed based on present and future trends and the market are estimated from 2024 to 2032.
Based on cancer type, the oncology biosimilar market is segmented as breast cancer, colorectal cancer, blood cancer, neutropenia cancer, non-small cell lung cancer and others.
On the basis of drug type, oncology biosimilar market is segmented into mAb, immunomodulators, hematopoietic agents, G-CSF, and others. G-CSF segment is expected to grow at the fastest rate during the forecast period.
Based on the distribution channel, oncology biosimilar market is segmented into retail pharmacies, hospital pharmacy, and online pharmacy. Hospital pharmacy segment is expected to grow at the fastest rate during the forecast period.Increasing demand for affordable therapies due to the constant increase in cancer patients is one of the major driving factors of oncology biosimilar market. High medical expenses associated with cancer treatment; the lack of awareness and dearth of skilled personnel are factors that influence the market growth during the forecast period.
Oncology Biosimilar Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Oncology Biosimilar Market Research Report |
| Market Size in 2023 | USD 6.13 Billion |
| Market Forecast in 2032 | USD 28.26 Billion |
| Growth Rate | CAGR of 18.50% |
| Number of Pages | 110 |
| Key Companies Covered | Teva Pharmaceutical Industries Ltd., Biocon, STADA Arzneimittel AG, Sandoz International GmbH, Intas Pharmaceuticals Ltd., Celltrion Inc., Pfizer Inc., Apotex Inc., Dr. Reddy’s Laboratories Ltd., BIOCAD, and among others. |
| Segments Covered | By Cancer Type, By Drug Type, By Distribution Channel, And By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Oncology Biosimilar Market: Regional Analysis
The regional segmentation includes the current and forecast demand for North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa with its further bifurcation into major countries, including the U.S., Canada, Mexico, Germany, Spain, Italy, France, UK, China, Japan, India, and Brazil. This segment includes demand for oncology biosimilar market based on individual segment and test types in all the regions and countries.
North America is expected to remain the dominant region over the forecast period which is closely followed by Europe. Due to the increased patent expiration and FDA approval of the new drugs the oncology biosimilar market is anticipated to grow rapidly over the forecast period. The Asia Pacific is expected to experience significant growth over the forecast period due to the increase in strategic collaboration in this region. Latin America and the Middle East & Africa are expected to experience a considerable growth over the forecast period. All the above-mentioned factors are responsible for the growth of oncology biosimilar market over the forecast period.
Oncology Biosimilar Market: Competitive Analysis
The report also includes detailed profiles of end players such as
- Teva Pharmaceutical Industries Ltd.
- Biocon
- STADA Arzneimittel AG
- Sandoz International GmbH
- Intas Pharmaceuticals Ltd.
- Celltrion Inc.
- Pfizer Inc.
- Apotex Inc.
- Dr. Reddy’s Laboratories Ltd.
- BIOCAD
- Among Others
The global oncology biosimilar market is segmented as follows:
By Cancer Type
- Breast Cancer
- Colorectal Cancer
- Blood Cancer
- Neutropenia Cancer
- Non-Small Cell Lung Cancer
- Others
By Drug Type
- mAb
- Immunomodulators
- Hematopoietic Agents
- G-CSF
- Others
By Distribution Channel
- Retail Pharmacies
- Hospital Pharmacy
- Online Pharmacy
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
An oncology biosimilar is a biological product that is highly similar to an already approved reference biologic drug used in the treatment of cancer.
According to study, the global oncology biosimilar market size was worth around USD 6.13 billion in 2023 and is predicted to grow to around USD 28.26 billion by 2032.
The CAGR value of oncology biosimilar market is expected to be around 18.50% during 2024-2032.
North America has been leading the global oncology biosimilar market and is anticipated to continue on the dominant position in the years to come.
The global oncology biosimilar market is led by players like Teva Pharmaceutical Industries Ltd., Biocon, STADA Arzneimittel AG, Sandoz International GmbH, Intas Pharmaceuticals Ltd., Celltrion Inc., Pfizer Inc., Apotex Inc., Dr. Reddy’s Laboratories Ltd., BIOCAD, and among others.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed