Paroxysmal Nocturnal Hemoglobinuria Drug Market Trend, Share, Growth, Size Analysis and Forecast 2030

Paroxysmal Nocturnal Hemoglobinuria Drug Market By Treatment (Medication, Supplements, Blood Transfusion, and Bone Marrow Transplant), By Drugs (Eculizumab, Ravulizumab, and Others), By Route of Administration (Oral and Parenteral), By Distribution Channel (Direct, Online Pharmacy, Retailers, and Others), By End User (Hospitals, Homecare, Specialty Clinics, and Others) and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2023 - 2030
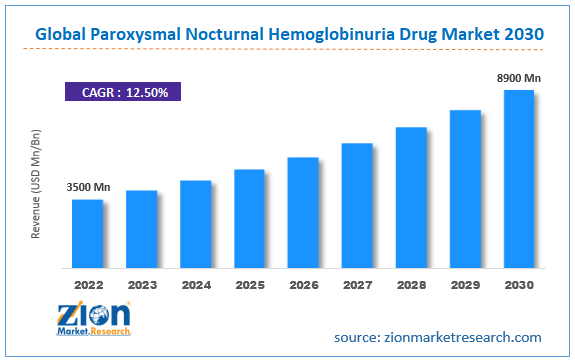
| Market Size in 2022 | Market Forecast in 2030 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 3.5 Billion | USD 8.9 Billion | 12.5% | 2022 |
Paroxysmal Nocturnal Hemoglobinuria Drug Industry Prospective:
The global paroxysmal nocturnal hemoglobinuria drug market size was worth around USD 3500 million in 2022 and is predicted to grow to around USD 8900 million by 2030 with a compound annual growth rate (CAGR) of roughly 12.5% between 2023 and 2030.
The report analyzes the global paroxysmal nocturnal hemoglobinuria drug market drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the paroxysmal nocturnal hemoglobinuria drug industry.
Paroxysmal Nocturnal Hemoglobinuria Drug Market: Overview
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare and potentially life-threatening blood disorder characterized by the destruction of red blood cells (hemolysis) and the presence of abnormal red blood cells in the bloodstream. It is caused by a genetic mutation that affects the proteins on the surface of red blood cells, leading to the excessive activation of the complement system, a part of the immune system responsible for destroying foreign substances in the body. The main goal of treatment for PNH is to control the symptoms and prevent complications. The introduction of targeted therapies has revolutionized the management of PNH. The most notable drug used for the treatment of PNH is eculizumab (brand name Soliris), which is a monoclonal antibody that inhibits the activation of the complement system. Eculizumab has been shown to be effective in reducing hemolysis, improving symptoms, and preventing complications such as thrombosis.
Eculizumab is administered intravenously every 1 to 2 weeks, depending on the patient's response and the severity of the disease. It has been approved by regulatory authorities for the treatment of PNH and has significantly improved the prognosis for individuals with this condition. Other treatment options may include bone marrow transplantation, which can potentially cure PNH, but it is considered a high-risk procedure and is generally reserved for severe cases or when other treatments have failed. The U.S. Food and Drug Administration (FDA) has approved an expanding number of paroxysmal nocturnal hemoglobinuria (PNH) treatments, including iron supplements, blood transfusions, blood thinners, eculizumab (Soliris), and bone marrow transplants. Additionally, new research and development projects are being carried out to create affordable paroxysmal nocturnal hemoglobinuria (PNH) treatments, which are anticipated to fuel the paroxysmal nocturnal hemoglobinuria drugs market's revenue.
Key Insights
- As per the analysis shared by our research analyst, the global paroxysmal nocturnal hemoglobinuria drug market is estimated to grow annually at a CAGR of around 12.5% over the forecast period (2023-2030).
- In terms of revenue, the global paroxysmal nocturnal hemoglobinuria drug market size was valued at around USD 3500 million in 2022 and is projected to reach USD 8900 million , by 2030.
- The increasing regulatory approvals for PNH drugs are expected to drive the growth of the paroxysmal nocturnal hemoglobinuria drug market during the forecast period.
- Based on the treatment, the blood transfusion segment is expected to capture the largest market share over the forecast period.
- Based on the end user, the hospital segment is expected to dominate the market during the forecast period.
- Based on region, North America is expected to hold the largest market share during the forecast period.
 Request Free Sample
Request Free Sample
Paroxysmal Nocturnal Hemoglobinuria Drug Market: Growth Drivers
Increasing regulatory approvals and R&D drive the market growth
Regulatory approvals by authorities such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other global regulatory bodies play a crucial role in the global paroxysmal nocturnal hemoglobinuria drug market dynamics. The approval of eculizumab and ravulizumab has provided a strong foundation for their market presence and increased accessibility for patients. Additionally, ongoing research and development efforts in the field of PNH are focused on developing new therapeutic options and improving existing treatments. This includes the development of novel targeted therapies, alternative administration methods, and exploring combination therapies. These advancements and innovations contribute to the growth and evolution of the PNH drug market.
Paroxysmal Nocturnal Hemoglobinuria Drug Market: Restraints
High treatment costs and limited patient population act as a major restraint for the market growth
The cost of targeted therapies for PNH, such as eculizumab and ravulizumab, can be substantial. These drugs require long-term administration, often for the patient's lifetime. The high treatment costs can pose financial challenges for patients, healthcare systems, and payers, potentially limiting access to these therapies and impacting the paroxysmal nocturnal hemoglobinuria drug industry growth. In addition, PNH is a rare disease, with a relatively small patient population. The limited number of patients with PNH can restrict the market size and limit the commercial viability of developing new drugs. The rarity of the disease also poses challenges for conducting clinical trials and generating robust evidence for new therapies.
Paroxysmal Nocturnal Hemoglobinuria Drug Market: Opportunities
The development of novel therapies offers a significant opportunity
Continued research and development efforts in PNH offer the opportunity to develop novel therapies that can further improve patient outcomes and address unmet needs. This includes exploring alternative mechanisms of action, combination therapies, and potentially curative treatments such as gene therapy or stem cell transplantation. The development of new drugs with enhanced efficacy, safety, and convenience can expand treatment options and drive the paroxysmal nocturnal hemoglobinuria drug industry growth.
Paroxysmal Nocturnal Hemoglobinuria Drug Market: Challenges
Regulatory and reimbursement barriers pose a major challenge to the market expansion
Regulatory approval processes and reimbursement policies can create barriers to PNH drugs. The complex and lengthy regulatory pathways for drug approval may delay access to innovative therapies. Reimbursement decisions by healthcare authorities and insurance companies can impact patient access to medications, especially in regions with strict cost-containment measures or limited healthcare resources. Thus, regulatory and reimbursement barriers are expected to hamper the growth of the paroxysmal nocturnal hemoglobinuria drug market during the forecast period.
Paroxysmal Nocturnal Hemoglobinuria Drug Market: Segmentation
The global paroxysmal nocturnal hemoglobinuria drug industry is segmented based on treatment, drugs, route of administration, distribution channel, end-user, and region.
Based on the treatment, the global market is bifurcated into medication, supplements, blood transfusion, and bone marrow transplants. The blood transfusion segment is expected to capture the largest market share over the forecast period. One of these treatments that might aid in easing anemia's symptoms is blood transfusion. PNH has serious side effects including thrombosis and can be lethal if left untreated. As a result, during a transfusion, the patients get blood or blood products from a donor, and PNH patients commonly need blood transfusions to address their anemic symptoms. The most frequent reason for continuous blood transfusion requirements in PNH patients utilizing eculizumab is chronic hemolysis with a persistently elevated reticulocyte count, and patients with PNH need transfusions using packed red blood cells rather than rescued autologous RBCs. PNH patients only use rescued autologous RBCs in critical situations, such as acute bleeding.
Based on the drugs, the global paroxysmal nocturnal hemoglobinuria drug industry is divided into eculizumab, ravulizumab, and others.
Based on the route of administration, the global market is divided into oral and parenteral.
Based on the distribution channel, the global paroxysmal nocturnal hemoglobinuria drug industry is segmented into direct, online pharmacies, retailers, and others.
Based on the end user, the global paroxysmal nocturnal hemoglobinuria drug industry is segmented into hospitals, homecare, specialty clinics, and others. The hospital segment is expected to dominate the market during the forecast period. Hospitals have hematologists on staff or at the very least PNH experts. These experts are more likely to be educated about this unusual ailment and stay current with PNH management and treatment breakthroughs. To quickly refer patients with vague symptoms to specialized clinics, several hospitals are creating fresh, enhanced methods to help in the early detection of PNH. Patients would be able to have timely access to excellent illness management by being properly evaluated and diagnosed. Additionally, they help to develop treatment plans that depend heavily on the patient's illness presentation and need interdisciplinary coordination, which considerably boosts the segment's revenue growth.
Paroxysmal Nocturnal Hemoglobinuria Drug Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Paroxysmal Nocturnal Hemoglobinuria Drug Market Research Report |
| Market Size in 2022 | USD 3500 Million |
| Market Forecast in 2030 | USD 8900 Million |
| Growth Rate | CAGR of 12.5% |
| Number of Pages | 214 |
| Key Companies Covered | Apellis Pharmaceuticals, Alexion Pharmaceuticals Inc, Akari Therapeutics Plc, CinnaGen Co, Ra Pharmaceuticals Inc, Amgen Inc, Achillion Pharmaceuticals Inc, Alnylam Pharmaceuticals Inc, F. Hoffmann-La Roche Ltd, Novartis AG, Regeneron Pharmaceuticals Inc, BIOCAD, Samsung Bioepis, Amyndas Pharmaceuticals, Teva Pharmaceutical Industries Ltd., LGM Pharma., Lannett, NorthStar Rx LLC, Abbott, Cook and Par Pharmaceutical among others. |
| Segments Covered | By Treatment, By Drugs, By Route of Administration, By Distribution Channel, By End User, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2022 |
| Historical Year | 2017 to 2021 |
| Forecast Year | 2023 - 2030 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Paroxysmal Nocturnal Hemoglobinuria Drug Market: Regional Analysis
North America is expected to hold the largest market share over the forecast period
North America is expected to hold the largest global paroxysmal nocturnal hemoglobinuria drug market share over the forecast period. The region's PNH drug market is dominated by major pharmaceutical companies that produce and market targeted therapies for PNH. Companies such as Alexion Pharmaceuticals (now part of AstraZeneca) and their drug eculizumab (trade name Soliris) have played a significant role in shaping the market landscape. In addition, the regulatory environment in North America, particularly the United States, plays a crucial role in the approval and accessibility of PNH drugs. The U.S. Food and Drug Administration (FDA) has granted approvals for targeted therapies like eculizumab and ravulizumab (trade name Ultomiris) for the treatment of PNH, contributing to their widespread use in the region.
Paroxysmal Nocturnal Hemoglobinuria Drug Market: Competitive Analysis
The global paroxysmal nocturnal hemoglobinuria drug market is dominated by players like:
- Apellis Pharmaceuticals
- Alexion Pharmaceuticals Inc
- Akari Therapeutics Plc
- CinnaGen Co
- Ra Pharmaceuticals Inc
- Amgen Inc
- Achillion Pharmaceuticals Inc
- Alnylam Pharmaceuticals Inc
- F. Hoffmann-La Roche Ltd
- Novartis AG
- Regeneron Pharmaceuticals Inc
- BIOCAD
- Samsung Bioepis
- Amyndas Pharmaceuticals
- Teva Pharmaceutical Industries Ltd.
- LGM Pharma.
- Lannett
- NorthStar Rx LLC
- Abbott
- Cook
- Par Pharmaceutical
The global paroxysmal nocturnal hemoglobinuria drug market is segmented as follows:
By Treatment
- Medication
- Supplements
- Blood Transfusion
- Bone Marrow Transplant
By Drugs
- Eculizumab
- Ravulizumab
- Others
By Route of Administration
- Oral
- Parenteral
By Distribution Channel
- Direct
- Online Pharmacy
- Retailers
- Others
By End User
- Hospitals
- Homecare
- Specialty Clinics
- Others
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare and potentially life-threatening blood disorder characterized by the destruction of red blood cells (hemolysis) and the presence of abnormal red blood cells in the bloodstream. It is caused by a genetic mutation that affects the proteins on the surface of red blood cells, leading to the excessive activation of the complement system, a part of the immune system responsible for destroying foreign substances in the body.
The U.S. Food and Drug Administration (FDA) has approved an increasing number of paroxysmal nocturnal hemoglobinuria (PNH) treatments, including iron supplements, blood transfusions, blood thinners, eculizumab (Soliris), and bone marrow transplants. As a result, the global market for PNH therapeutics is anticipated to expand significantly over the forecast period. The development of a low-cost paroxysmal nocturnal hemoglobinuria (PNH) medication is also the subject of recent research and development investigations, which is anticipated to fuel the market for therapies for PNH.
According to the report, the global paroxysmal nocturnal hemoglobinuria drug market size was worth around USD 3500 million in 2022 and is predicted to grow to around USD 8900 million by 2030.
What will be the CAGR value of the paroxysmal nocturnal hemoglobinuria drug market during 2023-2030?
The global paroxysmal nocturnal hemoglobinuria drug market is expected to grow at a CAGR of 12.5% during the forecast period.
The global paroxysmal nocturnal hemoglobinuria drug market growth is expected to be driven by North America. It is currently the world’s highest revenue-generating market owing to the presence of major players.
The global paroxysmal nocturnal hemoglobinuria drug market is dominated by players like Apellis Pharmaceuticals, Alexion Pharmaceuticals, Inc, Akari Therapeutics, Plc, CinnaGen Co, Ra Pharmaceuticals, Inc, Amgen Inc, Achillion Pharmaceuticals, Inc, Alnylam Pharmaceuticals, Inc, F. Hoffmann-La Roche Ltd, Novartis AG, Regeneron Pharmaceuticals, Inc, BIOCAD, Samsung Bioepis, Amyndas Pharmaceuticals, Teva Pharmaceutical Industries Ltd., LGM Pharma., Lannett, NorthStar Rx LLC, Abbott, Cook and Par Pharmaceutical among others.
Choose License Type
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed