Peripheral Stent Implants Market Size, Share, Growth, Forecast 2028

Peripheral Stent Implants Market By Product (Bare Metal Stents (BMS), Covered Stents, and Drug Eluting Stents (DES)), By Artery Type (Carotid Artery Stents, Fem-Pop Artery Stents, Iliac Artery Stents, and Infrapop Artery Stents), By End-User (Hospitals and Ambulatory Surgical Centers/Outpatient), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2022 - 2028
| Market Size in 2021 | Market Forecast in 2028 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 3291.5 million | USD 4857.1 million | 6.7% | 2021 |
Peripheral Stent Implants Market Size And Forecast
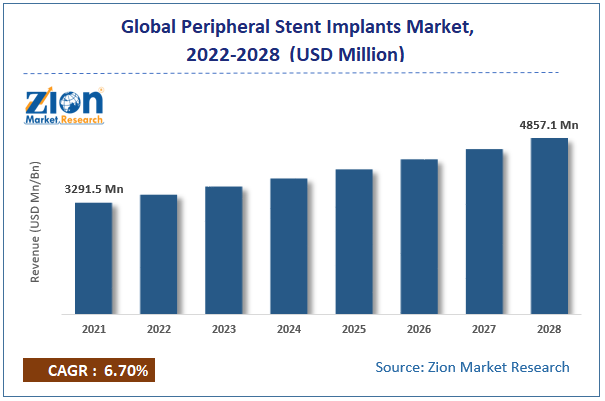
The global peripheral stent implants market size was worth USD 3,291.5 million in 2021 and is estimated to grow to USD 4,857.1 million by 2028, with a compound annual growth rate (CAGR) of roughly 6.7% over the forecast period.
The report analyzes the peripheral stent implants market's drivers, restraints/challenges, and their effect on the demands during the projection period. In addition, the report explores emerging opportunities in the peripheral stent implants market.
Peripheral Stent Implants Market: Overview
Fat deposits in the arteries lead to peripheral arterial disease, which reduces blood supply to the muscles. Conditions known as peripheral vascular diseases affect blood arteries not located in the heart, such as those in the brain, stomach, limbs, and kidneys. A vascular stent, also known as an intravascular stent, is a synthetic tubular mesh component placed permanently in the body's natural vasculature. Sales of peripheral stent implant devices are anticipated to increase due to increased awareness in low- and middle-income nations, growing government & industry investments to address the rising prevalence of peripheral artery disease, and technological advancements in vascular stents like drug-eluting & drug-coated stents.
Demand for peripheral stent implant devices is anticipated to increase as more people in these underserved markets have access to vascular stents for peripheral angioplasty and conventional open surgery. Other significant aspects expected to propel the worldwide market for peripheral stent implants expansion include rising end users and an increase in ambulatory surgery centers. The worldwide market for peripheral vascular stents is being constrained by strict restrictions placed on these devices to guarantee patient safety.
Key Insights
-
As per the analysis shared by our research analyst, the global peripheral stent implants market value is expected to grow at a CAGR of 6.7% over the forecast period.
- In terms of revenue, the global peripheral stent implants market size was valued at around USD 3,291.5 million in 2021 and is estimated to grow to USD 4,857.1 million by 2028.
- The market for peripheral stent implants is expanding due to the increased use of vascular stenting operations and the surge in demand for peripheral stents.
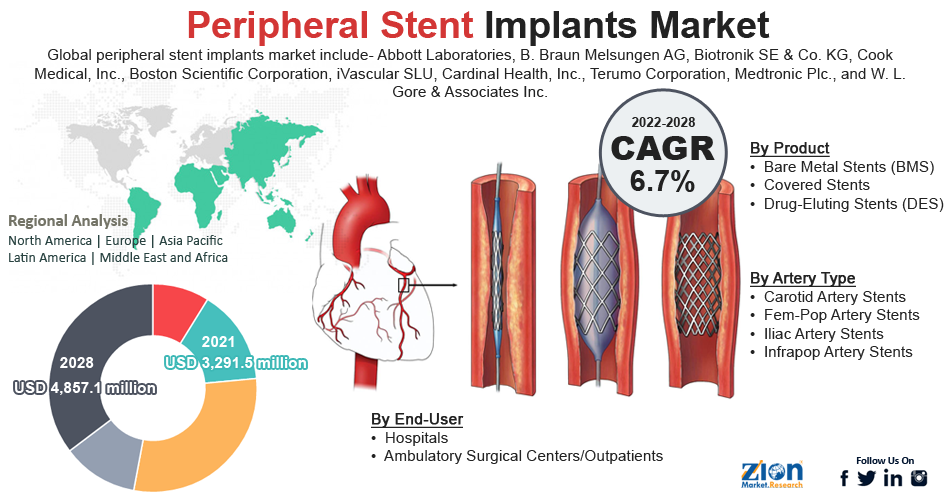
- By product, the drug-eluting stents (DES) category dominated the market in 2021.
- By artery type, the carotid artery stents category dominated the market in 2021.
- North America dominated the global peripheral stent implants market in 2021.
COVID-19 Impact:
Healthcare facilities in the area face unprecedented COVID-19-related operational and clinical difficulties. Additionally, a drop in market growth up to a certain level is being caused by the prioritization of COVID-19 diagnostic and treatment devices over cardiovascular treatment. Patients undergoing cardiac surgery not only need essential ICU services, but they also may be at the highest risk of COVID-19 consequences. Therefore, the majority of surgical facilities and hospitals have thought about canceling or delaying elective procedures, including cardiovascular procedures. Consequently, it is anticipated that the COVID-19 outbreak would have a mixed effect on the market for peripheral stent implant devices.
Peripheral Stent Implants Market: Growth Drivers
The increased prevalence of cardiovascular diseases drives the market growth
The expansion of the global peripheral stents implants market in the coming years is predicted to be favorably impacted by the rising prevalence of cardiovascular illnesses globally. Demand for peripheral vascular and percutaneous coronary interventions is anticipated to rise as the number of patients with chronic conditions rises. For instance, the World Health Organization (WHO) estimates that 17.9 million deaths worldwide each year are attributed to cardiovascular diseases (CVDs), or almost 32% of all fatalities. The need for peripheral vascular and percutaneous coronary interventions will rise as the number of patients with cardiovascular disorders increases gradually, which will ultimately feed the demand for peripheral stent implants.
Peripheral Stent Implants Market: Restraints
Strict regulatory requirements may hamper the growth of the market
Strict regulatory clearances may function as a roadblock to the expansion of the global peripheral stent implants market. For the effective and secure use of stents, several authorities and government organizations have established norms and recommendations. For instance, before approving stents, the U.S. FDA systematically examines the literature and animal pre-clinical trials. Additionally, because coronary stents fall within the FDA's category III of devices, approval of medical devices is thought to be resource-intensive (in terms of both time and money). Strict rules have been implemented with the difficulties that might arise from using stents. The U.S. FDA carefully supervises the use of stents due to potentially deadly effects. Certain stents are under class III medical devices, while others fall under class II, posing medium and severe risks to the patient. Since these gadgets must adhere to stringent regulatory requirements, the total market demand is hampered.
Peripheral Stent Implants Market: Segmentation
The global peripheral stent implants market has been segmented into product, artery type, end-user, and region.
Based on product, the worldwide peripheral stent implants market is segmented into bare metal stents (BMS), covered stents, and drug-eluting stents (DES). In 2021, the drug-eluting stents (DES) category dominated the global market. Strengthening DES's position as the standard device for PCI operations depends largely on the ongoing development and introduction of new devices. The market's rivals continue to create and introduce technologically sophisticated DES, with significant debuts, including XIENCE Skypoint by Abbott Laboratories, Resolute Onyx by Medtronic, and Synergy by Boston Scientific. These more recent DES offer superior stent integrity, higher deliverability, and fewer complication rates compared to older devices.
Based on artery type, the worldwide peripheral stent implants market is segmented into carotid artery stents, fem-pop artery stents, iliac artery stents, and infrapop artery stents. In 2021, the carotid artery stents category dominated the global market. The primary factors driving the carotid stents systems are the high prevalence of diseases like cardiovascular diseases, the large geriatric population undergoing bypass surgery, the rise in patient awareness programs, the surge in research & development initiatives for heart diseases, and the increase in demand for diagnostic imaging devices.
Based on end-user, the worldwide peripheral stent implants market is segmented into hospitals and ambulatory surgical centers/outpatients. In 2021, the hospital's category dominated the global market. Hospitals hold the largest market share for peripheral implant stents due to the accessibility of cutting-edge medical technology for treating artery-related conditions and peripheral vascular diseases. Medical equipment like stents, which are frequently utilized in a range of minimally invasive and surgical therapies, is readily available at hospitals. Growing disease populations result from various factors, including altered lifestyles, the adoption of unhealthy behaviors like drinking & smoking, and environmental factors like pollution. As a result, there will be a noticeable increase in hospital admissions, raising the need for better treatment options and opening up a sizable market for peripheral stent implant providers.
Recent Developments
-
In August 2021, Royal Philips, a global leader in health technology, announced that it had agreed to acquire Vesper Medical Inc., a medical technology business located in the United States that produces minimally invasive peripheral vascular devices. Vesper Medical will add an advanced venous stent portfolio for the treatment of deep venous illness to Philips' lineup of diagnostic and therapeutic devices. The deal is anticipated to close in the first quarter of 2022, subject to the usual closing requirements.
Peripheral Stent Implants Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Peripheral Stent Implants Market Research Report |
| Market Size in 2020 | USD 3,291.5 million |
| Market Forecast in 2028 | USD 4,857.1 million |
| Growth Rate | CAGR of 6.7% |
| Number of Pages | 210 |
| Key Companies Covered | Abbott Laboratories, B. Braun Melsungen AG, Biotronik SE & Co. KG, Cook Medical, Inc., Boston Scientific Corporation, iVascular SLU, Cardinal Health, Inc., Terumo Corporation, Medtronic Plc., and W. L. Gore & Associates Inc |
| Segments Covered | By Product, By Artery Type, By End User and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2021 |
| Historical Year | 2016 to 2020 |
| Forecast Year | 2020 - 2028 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Peripheral Stent Implants Market: Competitive Landscape
Some of the main competitors dominating the global peripheral stent implants market include-:
- Abbott Laboratories
- B. Braun Melsungen AG
- Biotronik SE & Co. KG
- Cook Medical, Inc.
- Boston Scientific Corporation
- iVascular SLU
- Cardinal Health, Inc.
- Terumo Corporation
- Medtronic Plc.
- W. L. Gore & Associates Inc.
Peripheral Stent Implants Market: Regional Landscape
North America dominated the peripheral stent implants market in 2021
In 2021, North America had a monopoly on the global peripheral stent implants market partly due to the adoption of recently authorized drug-eluting stents in the U.S. The region's market is expanding due to the aging population and rising adoption of minimally invasive procedures for cardiovascular problems. Additionally, significant market participants in the area guarantee that medical devices are broadly accessible. The International Journal of Epidemiology estimates that around 610,000 Americans lose their lives to one or more heart diseases yearly. In addition, around 735,000 Americans have a heart attack each year. Additionally, in recent years, the United States has approved new, technologically sophisticated stents, such as drug-eluting and bio-absorbable stents, which have completely changed the market for peripheral stent implants in the nation.
Global Peripheral Stent Implants Market is segmented as follows:
By Product
- Bare Metal Stents (BMS)
- Covered Stents
- Drug-Eluting Stents (DES)
By Artery Type
- Carotid Artery Stents
- Fem-Pop Artery Stents
- Iliac Artery Stents
- Infrapop Artery Stents
By End-User
- Hospitals
- Ambulatory Surgical Centers/Outpatients
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
The global market for peripheral stent implants is growing due to rising government & corporate support, more awareness in underdeveloped & emerging nations, among other factors. Additionally, it is anticipated that technological developments in stents and increased cases of peripheral artery disease would support market demand.
According to the report, the global peripheral stent implants market size was worth USD 3,291.5 million in 2021 and is estimated to grow to USD 4,857.1 million by 2028, with a compound annual growth rate (CAGR) of roughly 6.7% over the forecast period.
North America now holds a monopoly on the global market for peripheral stent implants due in part to the adoption of recently authorized drug-eluting stents in the U.S. The region's market is expanding due to aging populations, increased usage of peripheral stent implants, and rising adoption of minimally invasive procedures for cardiovascular problems.
Some of the main competitors dominating the global peripheral stent implants market include- Abbott Laboratories, B. Braun Melsungen AG, Biotronik SE & Co. KG, Cook Medical, Inc., Boston Scientific Corporation, iVascular SLU, Cardinal Health, Inc., Terumo Corporation, Medtronic Plc., and W. L. Gore & Associates Inc.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed