Pharmaceutical Solid Dosage Contract Manufacturing Market Size, Share, Trends, Growth 2030

Pharmaceutical Solid Dosage Contract Manufacturing Market By Product Type (Tablets, Capsule, Lozenge, Powder, And Others), By Drug Releases Pattern (Instant Release, Sustained Release, Extended Release, Effervescent Tablets, Chewable Tablets, Enteric Release), By Region- Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts, 2023 - 2030
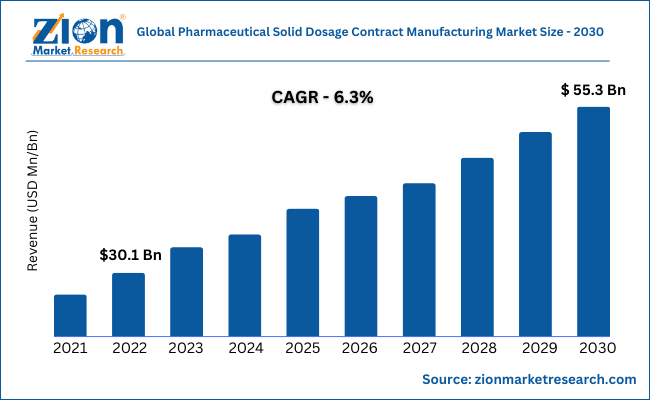
| Market Size in 2022 | Market Forecast in 2030 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 30.1 Billion | USD 55.3 Billion | 6.3% | 2022 |
Pharmaceutical Solid Dosage Contract Manufacturing Industry Perspective:
The global pharmaceutical solid dosage contract manufacturing market size was valued at USD 30.1 Billion in 2022 and is further predicted to reach USD 55.3 Billion by 2030, growing at a compound annual growth rate (CAGR) of 6.3% during the forecast period.
The market report offers quantitative and qualitative insights into the key drivers, opportunities, constraints, and challenges impacting global pharmaceutical solid dosage contract manufacturing industry growth.
Pharmaceutical Solid Dosage Contract Manufacturing Market: Overview
Pharmaceutical solid dosage forms are commonly used drug delivery methods across patient groups. Pharmaceuticals solid dosage contract manufacturing is dominating pharmaceutical formulations due to rising popularity and an increase in the adoption rate by pharmaceutical companies. The solid dosage formations are manufactured and are available in variants such as capsules, tablets, lozenges, etc.
The Pharmaceutical manufacturing unit is looking forward to outsourcing drug formulation, i.e., Contract Manufacturing Organization. A contract manufacturing organization (CMO) is an organization that serves the pharmaceutical industry and provides clients with comprehensive services, from drug development to manufacturing. Outsourcing to a CMO allows pharmaceutical clients to expand their technical resources, without the increased overhead and achieve economies of scale.
Key Insights
- As per the analysis shared by our research analyst, the global pharmaceutical solid dosage contract manufacturing market is expected to grow annually at a promising CAGR of around 6.3% during the forecast period 2023-2030.
- In terms of revenue, the global pharmaceutical solid dosage contract manufacturing market size was valued at around USD 30.1 billion in 2022 and is further predicted to reach 55.3 billion by 2030.
- Pharmaceutical solid dosage contract manufacturing involves outsourcing the manufacturing of tablets, capsules, and other solid dosage forms to third-party contract manufacturing organizations (CMOs). These CMOs specialize in the manufacturing, packaging, and labeling of pharmaceutical products for pharmaceutical companies. By outsourcing these services, pharmaceutical companies can focus on their core competencies, such as research and development, and reduce their capital expenditures.
- Based on product type, the tablets segment held the largest market share in 2022.
- Based on release pattern, the sustained release segment held the dominating market share in 2022.
- Based on region, the North American region held the largest market share in 2022.
Global Pharmaceutical Solid Dosage Contract Manufacturing Market: Growth Drivers
Increase in importance and investment toward healthcare drive the growth of the market
The pharmaceutical solid dosage provides improved efficiency and minimized cost of drug formulation, which is boosting the global pharmaceutical solid dosage contract manufacturing market growth. CMO (contract Manufacturing Outsourcing) will be beneficial for pharmaceutical companies due to the growing capital-intensive nature of the business as CMO will ensure business continuity and risk mitigation. Rising popularity, advanced technology, and increased governmental support are driving the pharmaceutical solid dosage contract manufacturing market globally.
Covid-19 has impacted the healthcare sector positively by shifting the focus of people toward the importance of healthcare. Government’s initiative to provide effective treatment to patients suffering from infection has proved to be a recreation of medical priorities across healthcare institutions. This will result in the growth of the Pharmaceutical Solid Dosage Contract Manufacturing market in the next coming years.
Global Pharmaceutical Solid Dosage Contract Manufacturing Market: Restraints
Prevalence of regulatory issues and supply chain disruptions might hinder the growth of the market
Potential restraints in the pharmaceutical solid dosage contract manufacturing industry include regulatory hurdles, increasing competition, pricing pressures, supply chain disruptions, and the need for significant investments in equipment and facilities. Additionally, the increasing complexity of drug formulations and the need for specialized expertise may make it difficult for some contract manufacturers to keep up with the demands of the market.
Global Pharmaceutical Solid Dosage Contract Manufacturing Market: Opportunities
Persistent technological advancements and rise in need for cost-effective solutions create ample opportunities for the market
The global pharmaceutical solid dosage contract manufacturing market presents several opportunities for growth. The increasing demand for personalized medicine and the need for specialized formulations has created a niche for contract manufacturers with expertise in these areas.
Additionally, the trend towards outsourcing and the need for cost-effective solutions by pharmaceutical companies provide opportunities for contract manufacturers to expand their client base. The growing importance of biologics and biosimilars in the pharmaceutical industry also presents an opportunity for contract manufacturers to develop and expand their capabilities in this area. Finally, advancements in technology, such as automation and continuous manufacturing, provide opportunities for contract manufacturers to improve efficiency and reduce costs.
Global Pharmaceutical Solid Dosage Contract Manufacturing Market: Challenges
Apprehensions regarding risk management with intellectual property and competitiveness of the industry might act as a challenge
The pharmaceutical solid dosage contract manufacturing industry faces several challenges. One of the main challenges is ensuring compliance with regulatory requirements, which can be time-consuming and costly. The industry is also highly competitive, and contract manufacturers must be able to provide high-quality services at competitive prices. Other challenges include maintaining a reliable supply chain, managing risks associated with intellectual property, and staying up-to-date with new technologies and industry developments. The increasing demand for flexibility and customization in manufacturing processes also poses a challenge for contract manufacturers.
Global Pharmaceutical Solid Dosage Contract Manufacturing Market: Segmentation
The global pharmaceutical solid dosage contract manufacturing market is bifurcated into product type, drug release pattern, and region.
Based on product type, the market is bifurcated into tablets, capsules, powder, lozenge, and others. The tablets segment held the largest market share in 2022 and is further predicted to grow rapidly at a remarkable CAGR during the forecast period. This is mainly due to the increased popularity of tablets as a preferred dosage form by patients due to their ease of administration, portability, and convenience. Tablets also offer better stability and longer shelf life compared to other solid dosage forms, making them an attractive option for pharmaceutical companies. Additionally, tablets can be produced at a larger scale, allowing for cost savings through economies of scale. These factors drive the growth of the segment during the forecast period.
Based on drug release pattern, the market is segmented into instant release, sustained release, extended release, effervescent tablets, chewable tablets, and enteric release. The sustained release segment held the dominating market share in 2022 and is further predicted to cite the fastest CAGR during the forecast period. The growth of the segment is mainly due to increasing demand for medications that provide a slow and steady release of the active ingredient over an extended period. Sustained-release formulations allow for reduced dosing frequency, better patient compliance, and improved therapeutic outcomes.
These formulations are particularly useful for chronic conditions that require continuous medication and where it may be difficult for patients to adhere to a dosing regimen. In addition, sustained-release formulations can also reduce the risk of side effects and toxicity associated with high doses of medication, as they release the active ingredient slowly over time. This has led to increased interest from pharmaceutical companies and healthcare providers in developing sustained-release formulations for a variety of therapeutic areas, including cardiovascular disease, diabetes, and pain management. These factors further drive the growth of the segment during the forecast period.
Recent Developments
- In November 2021, Catalent announced the acquisition of RheinCell Therapeutics, a German-based cell therapy company. The acquisition will help Catalent expand its capabilities in the cell and gene therapy space, which is a growing area of the pharmaceutical industry. Additionally, in January 2022, Catalent announced plans to invest $100 million in expanding its biologics manufacturing facility in Wisconsin, USA. This expansion will help Catalent meet the increasing demand for biologics manufacturing services.
- In September 2021, Lonza announced the expansion of its cell and gene therapy manufacturing facility in Pearland, Texas. The expansion will double the facility's manufacturing capacity and is expected to be completed in 2023. Additionally, in October 2021, Lonza announced a collaboration with Cryoport, a logistics company specializing in cell and gene therapies, to provide end-to-end supply chain solutions for the industry.
Global Pharmaceutical Solid Dosage Contract Manufacturing Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Pharmaceutical Solid Dosage Contract Manufacturing Market Research Report |
| Market Size in 2022 | USD 30.1 Billion |
| Market Forecast in 2030 | USD 55.3 Billion |
| Growth Rate | CAGR of 6.3% |
| Number of Pages | 190 |
| Key Companies Covered | HAUPT Pharma AG, Abbott Laboratories, NextPharma, Catalent Pharma Solutions, Althea Technologies, Jubilant Life Sciences Limited, Royal DSM N.V and Nipro Corp. Aenova, Alkermes plc, Associates of Cape Cod Inc., BioPharma Solutions, , Coldstream Laboratories Inc., Covance Inc., Cytovance Biologics, Inc., Dalton Pharma Services, DPT Laboratories, Emergent BioSolutions Inc., Fresenius Kabi, Grand River Aseptic Manufacturing (GRAM), Halo Pharmaceutical, IGI Laboratories, Lyophilization Technology, Inc., Metrics Inc., Mikart, Inc., Patheon, Inc., Pillar5 Pharma Inc., and Velesco Pharma, etc. |
| Segments Covered | By Product Type, By Drug Releases Pattern, And By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2022 |
| Historical Year | 2017 to 2021 |
| Forecast Year | 2023 - 2030 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Global Pharmaceutical Solid Dosage Contract Manufacturing Market: Regional Analysis
North America to lead the global market followed by Europe
The global pharmaceutical solid dosage contract manufacturing market is dominated by North America followed by Europe due to driving factors such as advancement in technology, improved healthcare infrastructure, and increased research & development activities in the market of pharmaceuticals. CMO has also contributed to the region’s market by investing in new technologies and facilities by introducing distributed manufacturing to take manufacturing closer to the patient and adopting continuous manufacturing & digitization of processes.
For example, CMOs are partnering with third-party vendors for layered analytics with the aim of optimizing processes, introducing preventive maintenance, and making supply chains transparent. Rising number of companies outsourcing projects in the developing economies of this region has also ensured the growth of the market. Owing to the increased initiatives by the Asia Pacific government and the adoption of advanced technologies, the pharmaceutical solid dosage contract manufacturing market will be growing rapidly in Asia Pacific in the next coming years.
Global Pharmaceutical Solid Dosage Contract Manufacturing Market: Competitive Players
Key players in the global pharmaceutical solid dosage contract manufacturing market include:
- HAUPT Pharma AG
- Abbott Laboratories
- NextPharma
- Catalent Pharma Solutions
- Althea Technologies
- Jubilant Life Sciences Limited
- Royal DSM N.V and Nipro Corp. Aenova
- Alkermes plc
- Associates of Cape Cod Inc.
- BioPharma Solutions
- Coldstream Laboratories Inc.
- Covance Inc.
- Cytovance Biologics Inc.
- Dalton Pharma Services
- DPT Laboratories
- Emergent BioSolutions Inc.
- Fresenius Kabi
- Grand River Aseptic Manufacturing (GRAM)
- Halo Pharmaceutical
- IGI Laboratories
- Lyophilization Technology Inc.
- Metrics Inc.
- Mikart Inc.
- Patheon Inc.
- Pillar5 Pharma Inc.
- Velesco Pharma
The global pharmaceutical solid dosage contract manufacturing market is segmented as follows:
By Product Type
- Tablets
- Capsule
- Lozenge
- Powder
By Drug Releases Pattern
- Instant Release
- Sustained Release
- Extended Release
- Effervescent Tablets
- Chewable Tablets
- Enteric Release
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Pharmaceutical solid dosage forms are commonly used drug delivery methods across patient groups. The Pharmaceutical manufacturing unit is looking forward to outsourcing drug formulation, i.e., Contract Manufacturing Organization. A contract manufacturing organization (CMO) is an organization that serves the pharmaceutical industry and provides clients with comprehensive services, from drug development to manufacturing. Outsourcing to a CMO allows pharmaceutical clients to expand their technical resources, without the increased overhead and achieve economies of scale.
The global pharmaceutical solid dosage contract manufacturing market is predicted to increase at a CAGR of 6.3% during the forecast period.
The global pharmaceutical solid dosage contract manufacturing market was worth around USD 30.1 billion in 2022 and is further predicted to reach 55.3 billion by 2030.
The global pharmaceutical solid dosage contract manufacturing market is being driven by several factors, including the increasing demand for pharmaceuticals, the need for cost-effective solutions, advancements in technology, the trend toward outsourcing, and the growing importance of specialized formulations and personalized medicine.
North America held the largest share in the global pharmaceutical solid dosage contract manufacturing market in 2022 and is likely to continue the same trend during the forecast period. The growth of the market in North America is being driven by factors such as increasing demand for pharmaceuticals, the presence of a well-established pharmaceutical industry, favorable government initiatives, and a growing trend toward outsourcing and cost-effective solutions. The region also has a strong focus on research and development, which further supports market growth.
Some of the major companies operating in the pharmaceutical solid dosage contract manufacturing market include HAUPT Pharma AG, Abbott Laboratories, NextPharma, Catalent Pharma Solutions, Althea Technologies, Jubilant Life Sciences Limited, Royal DSM N.V and Nipro Corp. Aenova, Alkermes plc, Associates of Cape Cod Inc., BioPharma Solutions, Coldstream Laboratories Inc., Covance Inc., Cytovance Biologics, Inc., Dalton Pharma Services, DPT Laboratories, Emergent BioSolutions Inc., Fresenius Kabi, Grand River Aseptic Manufacturing (GRAM), Halo Pharmaceutical, IGI Laboratories, Lyophilization Technology, Inc., Metrics Inc., Mikart, Inc., Patheon, Inc., Pillar5 Pharma Inc., and Velesco Pharma, etc.
Choose License Type
List of Contents
Manufacturing Industry Perspective: Manufacturing OverviewKey InsightsGlobal Manufacturing Growth DriversGlobal Manufacturing RestraintsGlobal Manufacturing OpportunitiesGlobal Manufacturing ChallengesGlobal Manufacturing SegmentationRecent DevelopmentsGlobal Manufacturing Report ScopeGlobal Manufacturing Regional AnalysisGlobal Manufacturing Competitive PlayersThe global pharmaceutical solid dosage contract manufacturing market is segmented as follows:RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed