Reprocessed Medical Device Market Size, Share Report, Analysis, Trends, Growth 2032

Reprocessed Medical Device Market Size - By Device (Cardiovascular Medical Devices, Gastroenterology and Urology, Orthopedic/Arthroscopic, Laparoscopic, General Surgery Equipment), By End-User (Hospitals, Clinics, Ambulatory Surgical Centre, Other End Users), By Region - Global And Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, And Forecasts 2024 - 2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 2.69 Billion | USD 10.57 Billion | 16.43% | 2023 |
Reprocessed Medical Device Industry Prospective:
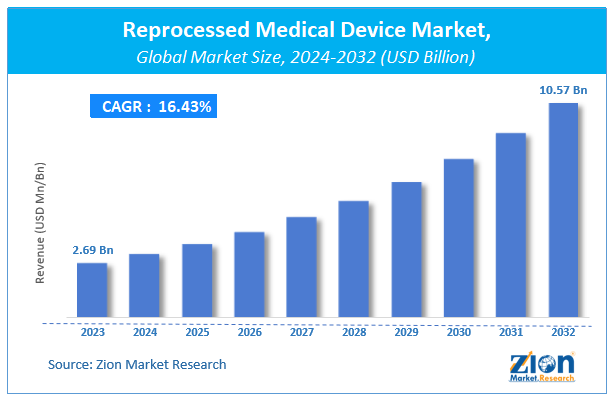
The reprocessed medical device market size was worth around USD 2.69 Million in 2023 and is predicted to grow to around USD 10.57 Million by 2032 with a compound annual growth rate (CAGR) of roughly 16.43% between 2024 and 2032.
The report covers forecast and analysis for the reprocessed medical device market on a global and regional level. The study provides historical data from 2018 to 2022 along with a forecast from 2024 to 2032 based on revenue (USD Million). The study includes drivers and restraints for the reprocessed medical device market along with the impact they have on the demand over the forecast period. Additionally, the report includes the study of opportunities and various trends in the reprocessed medical device market on a global level.
Reprocessed Medical Device Market: Overview
A reprocessed medical device is a medical instrument or piece of equipment that has been previously used, thoroughly cleaned, sterilized, and refurbished to meet safety and performance standards, allowing it to be reused in clinical settings. The reprocessing process involves several critical steps, including the removal of contaminants, thorough cleaning, sterilization to eliminate microorganisms, and rigorous inspection and testing to ensure the device's functionality and safety. This practice helps reduce medical waste and lowers healthcare costs, making it an economically and environmentally advantageous alternative to disposing of single-use devices. Reprocessed medical devices are subjected to stringent regulatory oversight to ensure that they meet the same safety and efficacy standards as new devices, providing a viable option for extending the life of expensive medical equipment.
Reprocessed Medical Device Market: Growth Factors
A reprocessed medical device is a device that is being refurbished and reused. Medical device reprocessing involves disinfection, remanufacturing, testing, sterilization, cleaning, packaging and labeling of the devices in order to be put back into service. Any medical device must be reprocessed through a validated method that makes it suitable for use. Consideration is given to reprocessing device variables such as efficacy, life cycle, prospective danger to the patient and parts used to make the device. With rising healthcare costs and elevated prices of medical devices, demand for reprocessed medical devices over the forecast period is anticipated to experience elevated development. Increasing incidences of chronic conditions that increase the rate of implementation of surgical procedures show important development in the market for reprocessed medical devices. Reprocessed device costs can be almost half the cost of a fresh device; opting for reprocessed systems is cost-effective. This means that reprocessed devices cost less than fresh appliances, leading in hospital cost reserves that stimulate market growth.
Reprocessed devices can include a variety of items, such as surgical instruments, endoscopes, and other high-cost items that, if properly reprocessed, can be safely used multiple times. The market for reprocessed medical devices is growing due to increasing healthcare costs, a push for sustainability, and the need to maximize the utilization of expensive medical equipment. Regulatory bodies, such as the FDA, have established guidelines to ensure that reprocessed devices meet safety and efficacy standards, providing assurance to healthcare providers and patients regarding their use.
The market growth is driven by factors such as advancements in reprocessing technologies, increasing healthcare expenditure, and the growing emphasis on environmental sustainability. However, challenges like regulatory hurdles, quality control concerns, and resistance from some healthcare providers regarding the safety of reprocessed devices can impact market growth. Overall, the reprocessed medical device market plays a crucial role in promoting cost-effective and environmentally friendly practices within the healthcare sector.
Reprocessed Medical Device Market: Segmentation
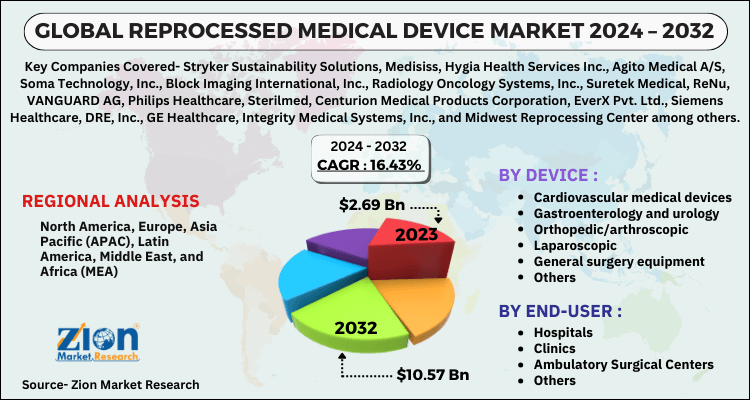
Based on device, the reprocessed medical device market is segmented into cardiovascular medical devices, gastroenterology and urology, orthopedic/arthroscopic, laparoscopic, general surgery equipment and others. Due to the comprehensive use of these goods in cardiac surgery and diagnostics, the cardiovascular segment retained the biggest share of the complete market. The instruments include blood pressure cuffs, devices for cardiac placement and stability, tracking of blood pressure, electrophysiology wires and catheters for diagnostic electrophysiology.
The reprocessed medical device market is further divided into end users as hospitals, ASCs, clinics, and others. Hospitals application segment dominated the market in terms of revenue in 2023 attributing to the fact that the patients have more inclination towards hospitals and to manage such a large patient pool, hospitals need more reprocessed medical devices.
Reprocessed Medical Device Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Reprocessed Medical Device Market |
| Market Size in 2023 | USD 2.69 Billion |
| Market Forecast in 2032 | USD 10.57 Billion |
| Growth Rate | CAGR of 16.43% |
| Number of Pages | 110 |
| Key Companies Covered | Stryker Sustainability Solutions, Medisiss, Hygia Health Services Inc., Agito Medical A/S, Soma Technology, Inc., Block Imaging International, Inc., Radiology Oncology Systems, Inc., Suretek Medical, ReNu, VANGUARD AG, Philips Healthcare, Sterilmed, Centurion Medical Products Corporation, EverX Pvt. Ltd., Siemens Healthcare, DRE, Inc., GE Healthcare, Integrity Medical Systems, Inc., and Midwest Reprocessing Center among others |
| Segments Covered | By Device, By End User and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Reprocessed Medical Device Market: Regional Insights
In 2023, the North America dominated the reprocessed medical devices industry and will continue to develop considerably over the forecast period .A significant driver in this region is the growing need to curtail waste generation and potential environmental harm due to the growing waste landfills across this region. Increasing demand to reduce healthcare spending and the shortage of adequate waste disposal facilities are driving this region's development. Europe is followed in terms of revenue sharing due to elevated rates of awareness and initiatives to reduce general healthcare spending across this region. Organizations such as the European Medical Device Reprocessing Association (EAMDR), encourage awareness by working with research institutes, businesses and associations to enhance product reprocessing. Because of the existence of emerging economies, which concentrate on functioning within a regulated budget, Asia Pacific is expected to develop at a profitable pace. Furthermore, increasing investment in healthcare infrastructure by global market players is expected to provide potential growth platform to the industry in Asia Pacific.
Reprocessed Medical Device Market: Competitive Space
Some of the key players in reprocessed medical device market include
- Stryker Sustainability Solutions
- Medisiss
- Hygia Health Services Inc.
- Agito Medical A/S
- Soma Technology. Inc.
- Block Imaging International. Inc.
- Radiology Oncology Systems. Inc.
- Suretek Medical
- ReNu
- VANGUARD AG
- Philips Healthcare
- Sterilmed
- Centurion Medical Products Corporation
- EverX Pvt. Ltd.
- Siemens Healthcare
- DRE. Inc.
- GE Healthcare
- Integrity Medical Systems. Inc.
- Midwest Reprocessing Center
- among others.
The global Reprocessed Medical Device market is segmented as follows:
Global Reprocessed Medical Device Market: Device Segment Analysis
- Cardiovascular medical devices
- Gastroenterology and urology
- Orthopedic/arthroscopic
- Laparoscopic
- General surgery equipment
- Others
Global Reprocessed Medical Device Market: End User Segment Analysis
- Hospitals
- Clinics
- Ambulatory Surgical Centers
- Others
Global Reprocessed Medical Device Market: Regional Segment Analysis
- North America
- U.S.
- Europe
- UK
- France
- Germany
- Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- Middle East and Africa
Table Of Content
Methodology
FrequentlyAsked Questions
The Reprocessed Medical Device Market involves the segment of the healthcare industry focused on the collection, cleaning, sterilization, and reuse of medical devices that are intended for single use. Reprocessed medical devices are typically those that have been used once, then undergo a rigorous process to restore them to a condition suitable for reuse in medical procedures. This process helps in reducing medical waste and lowering healthcare costs.
A reprocessed medical device is a device that is being refurbished and reused. Medical device reprocessing involves disinfection, remanufacturing, testing, sterilization, cleaning, packaging and labeling of the devices in order to be put back into service. Any medical device must be reprocessed through a validated method that makes it suitable for use. Consideration is given to reprocessing device variables such as efficacy, life cycle, prospective danger to the patient and parts used to make the device. With rising healthcare costs and elevated prices of medical devices, demand for reprocessed medical devices over the forecast period is anticipated to experience elevated development.
According to a study, the global reprocessed medical device Industry size was $2.69 million in 2023 and is projected to reach $10.57 million by the end of 2032.
The global reprocessed medical device market is expected to grow at a CAGR of 16.43% during the forecast period.
The North America dominated the reprocessed medical devices industry and will continue to develop considerably over the forecast period.
Some of the key players in reprocessed medical device market include Stryker Sustainability Solutions, Medisiss, Hygia Health Services Inc., Agito Medical A/S, Soma Technology, Inc., Block Imaging International, Inc., Radiology Oncology Systems, Inc., Suretek Medical, ReNu, VANGUARD AG, Philips Healthcare, Sterilmed, Centurion Medical Products Corporation, EverX Pvt. Ltd., Siemens Healthcare, DRE, Inc., GE Healthcare, Integrity Medical Systems, Inc., and Midwest Reprocessing Center among others.
The global reprocessed medical device market report covers the geographical market along with a comprehensive competitive landscape analysis. It also includes cash flow analysis, profit ratio analysis, market basket analysis, market attractiveness analysis, sentiment analysis, PESTEL analysis, trend analysis, SWOT analysis, trade area analysis, demand & supply analysis, Porter’s five force analysis, and value chain analysis.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed