Scleroderma Diagnostics and Therapeutics Market Size, Global Report 2034

Scleroderma Diagnostics and Therapeutics Market By Diagnostic Test Type (Blood Tests, Imaging Techniques, Skin Biopsy, Pulmonary Function Tests, and Electrocardiogram & Echocardiogram), By Indication (Systemic Scleroderma and Localized Scleroderma), By Drug Class (Prostacyclin Analogues, Endothelin Receptor Antagonists, Phosphodiesterase 5 Inhibitors - PHA, Immunosuppressors, Analgesics, Calcium Channel Blockers, and Others), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 2.52 Billion | USD 3.98 Billion | 5.2% | 2024 |
Scleroderma Diagnostics and Therapeutics Industry Perspective:
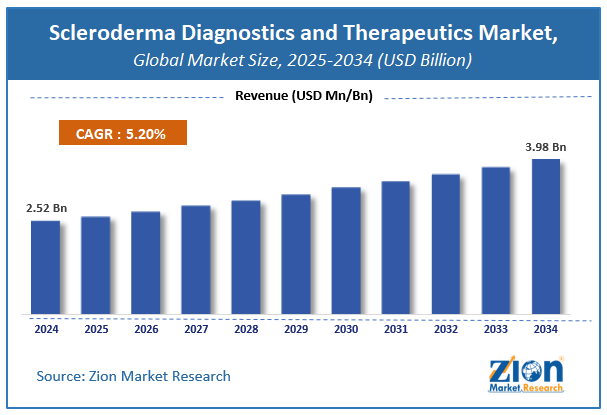
The global scleroderma diagnostics and therapeutics market size was worth around USD 2.52 Billion in 2024 and is predicted to grow to around USD 3.98 Billion by 2034 with a compound annual growth rate (CAGR) of roughly 5.2% between 2025 and 2034. The report analyzes the global scleroderma diagnostics and therapeutics market's drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the scleroderma diagnostics and therapeutics industry. The report studies the scleroderma diagnostics and therapeutics market’s drivers, restraints/challenges, and the consequence they have on the demands during the projection period. In addition, the report sightsees evolving opportunities in the scleroderma diagnostics and therapeutics market.
Scleroderma Diagnostics and Therapeutics Market: Overview
Scleroderma is an autoimmune disease wherein healthy tissue is substituted with thick, dense, fibrous tissue. It is a chronic, albeit rare, condition. The immune system normally aids in the body's defense against sickness and infection. The immune system in scleroderma patients causes other cells to create excessive collagen. This excess collagen builds up in the organs and skin, causing hardness and thickness similar to the scarring process.
While scleroderma has no cure, therapy can help to alleviate symptoms, reduce progression, and enhance the quality of life. Scleroderma can impact several other body parts, such as the gastrointestinal system, joints, muscles, blood vessels, kidneys, lungs, and skin. Scleroderma can be life-threatening in its most severe forms.
Key Insights
- As per the analysis shared by our research analyst, the global scleroderma diagnostics and therapeutics market is estimated to grow annually at a CAGR of around 5.2% over the forecast period (2025-2034).
- Regarding revenue, the global scleroderma diagnostics and therapeutics market size was valued at around USD 2.52 Billion in 2024 and is projected to reach USD 3.98 Billion by 2034.
- The scleroderma diagnostics and therapeutics market is projected to grow at a significant rate due to rising prevalence of scleroderma, increasing awareness and early diagnosis, advancements in targeted therapies, and growing research in autoimmune disease treatment.
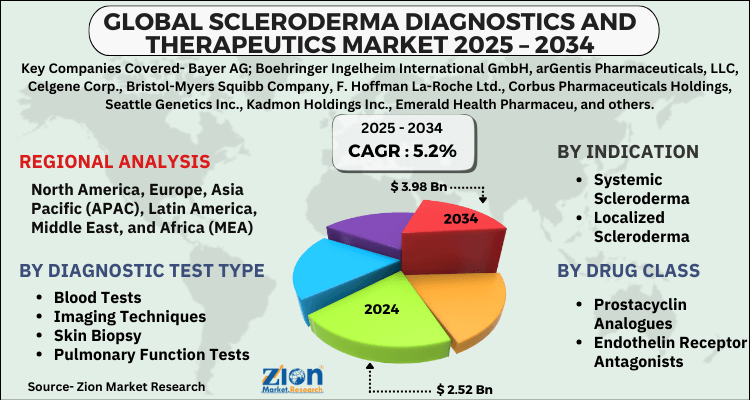
- Based on Diagnostic Test Type, the Blood Tests segment is expected to lead the global market.
- On the basis of Indication, the Systemic Scleroderma segment is growing at a high rate and will continue to dominate the global market.
- Based on the Drug Class, the Prostacyclin Analogues segment is projected to swipe the largest market share.
- Based on region, North America is predicted to dominate the global market during the forecast period.
Scleroderma Diagnostics and Therapeutics Market: Growth Drivers
Growing prevalence of scleroderma is fostering the growth of the market.
In developed and developing countries, the prevalence of scleroderma is growing at a rapid rate. This condition affects between 75,000 to 100,000 persons in the United States, the majority of whom are females between the ages of 30 to 50. Twins and family members of people with scleroderma or even other autoimmune connective tissue illnesses like lupus are at an increased risk of developing the condition. Scleroderma can affect children, although the condition affects them differently than it does adults.
Such a huge burden of disease has led to increase in awareness and need for diagnosis of the disease. This is ultimately driving the market growth. In addition to this, favorable reimbursement policies, well-developed health infrastructure, and growing necessity for developed diagnostic techniques are some of the key factors that are boosting the growth of the market for scleroderma diagnostics and therapeutics.
Scleroderma Diagnostics and Therapeutics Market: Restraints
High cost associated with scleroderma diagnostic and treatment is likely to hamper the market growth.
The multi-organ nature of systemic sclerosis necessitates long-term monitoring and a variety of approaches to patient management. As a result, it's no surprise that systemic sclerosis is one of the most expensive rheumatic illnesses, with patients using more healthcare dollars per year than their age and sex-matched colleagues with inflammatory myopathies, and psoriatic arthritis, and/or RA. Additionally, some of the direct costs for patients with systemic sclerosis include acute-care hospitalization, assistive devices, medications, diagnostic tests as well as visits to healthcare professionals.
Thus, the financial cost of systemic sclerosis is substantial, and disease & health conditions have a significant influence on the economic burden thereby impeding the growth of the global scleroderma diagnostics and therapeutics market. In addition to this, factors such as low penetration of the market in underdeveloped countries, adverse effects associated with the drugs, and low spending power in rural areas are also hampering the market growth.
Scleroderma Diagnostics and Therapeutics Market: Opportunities
Growing focus on development of innovative methods is estimated to drive the market during the forecast period.
Systemic sclerosis is a multifaceted autoimmune disease with a wide range of symptoms and degrees of internal organ damage. All consequences have a substantial influence on the patient's quality of life as well as morbidity and death rates. As a result, there is a growing interest in novel ways of assessing, diagnosing, and monitoring organ involvement. In addition to this, there is an increase in investment in R&D activities by major players for the development of new and effective treatments.
Major players are also focusing on the completion of clinical trials for the drugs and looking forward to the approval of the drug for the treatment of scleroderma. All such factors coupled with technological advancements including the implementation of artificial intelligence and internet of things in the field of medical devices are anticipated to generate numerous opportunities for the growth of the global scleroderma diagnostics and therapeutics market during the forecast period.
Scleroderma Diagnostics and Therapeutics Market: Challenges
Strict regulations for the approval of drugs and diagnostic devices for scleroderma may act as a challenge to the market growth
By far there are very less approved drugs for the treatment of scleroderma. Scleroderma is a rare disease and its diagnosis is also challenging. The vague clinical presentation of scleroderma might make diagnosis difficult, resulting in a delayed diagnosis. This also interferes in the development of drugs that can be effectively used to treat the disease. The lack of sufficient data on the efficacy and safety of the drugs leads to the failure of clinical trials.
The regulatory bodies across different countries have imposed strict regulations for the approval of drugs and diagnostic devices in order to have appropriate diagnosis and treatment of the disease. This may act as a major challenge to the growth of the market.
Scleroderma Diagnostics and Therapeutics Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Scleroderma Diagnostics and Therapeutics Market |
| Market Size in 2024 | USD 2.52 Billion |
| Market Forecast in 2034 | USD 3.98 Billion |
| Growth Rate | CAGR of 5.2% |
| Number of Pages | 180 |
| Key Companies Covered | Bayer AG; Boehringer Ingelheim International GmbH, arGentis Pharmaceuticals, LLC, Celgene Corp., Bristol-Myers Squibb Company, F. Hoffman La-Roche Ltd., Corbus Pharmaceuticals Holdings, Seattle Genetics Inc., Kadmon Holdings Inc., Emerald Health Pharmaceu, and others. |
| Segments Covered | By Diagnostic Test Type, By Indication, By Drug Class, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, The Middle East and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2020 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Scleroderma Diagnostics and Therapeutics Market: Segmentation
The global scleroderma diagnostic and therapeutics market is categorized based on diagnostic test type, indication, drug class, and region.
The diagnostic test type, segment comprises blood tests, imaging techniques, skin biopsy, pulmonary function tests, and electrocardiogram & echocardiogram.
Based on the indication, the global market is bifurcated into systemic scleroderma and localized scleroderma.
The drug class segment, is classified into prostacyclin analogues, endothelin receptor antagonists, phosphodiesterase 5 inhibitors – PHA, immunosuppressors, analgesics, calcium channel blockers, and others.
Recent Developments
- In March 2021, Roche received approval for Actemra®/RoActemra® (tocilizumab) subcutaneous injection from the US Food and Drug Administration (FDA) for decreasing the rate of drop in pulmonary function in adult patients with systemic sclerosis-associated interstitial lung disease (SSc-ILD), a chronic condition with few treatment options. The FDA has approved Actemra/RoActemra as the first biologic medication for the treatment of the condition.
- In April 2020, Boehringer Ingelheim received approval from the European Commission for nintedanib for the treatment of people with systemic sclerosis-associated interstitial lung disease (SSc-ILD).
Scleroderma Diagnostics and Therapeutics Market: Regional Landscape
North America is estimated to rule the market for scleroderma diagnostics and therapeutics
Among the regions, North America is expected to dominate the global scleroderma diagnostics and therapeutics market. Major factors such as favorable reimbursement policies, heavy investment in R&D and drug development, and well-established healthcare infrastructure are boosting the growth of the market in this region. Additionally, the growing prevalence of the disease and technological advancement in the field of medical devices are also propelling the growth of the market in this region.
Europe is anticipated to hold the second-most position in the market owing to an increase in initiatives and proactive awareness regarding diagnostics for scleroderma. On the other hand, the market in Asia Pacific is likely to grow at the highest CAGR during the forecast period. This is majorly due to the wide and easy availability of generic & biosimilar immunosuppressants for the treatment of scleroderma.
Scleroderma Diagnostics and Therapeutics Market: Competitive Landscape
The report provides a company market share analysis to give a broader overview of the key market players. In addition, the report also covers key strategic developments of the market, including acquisitions & mergers, new product launches, agreements, partnerships, collaborations & joint ventures, research & development, and regional expansion of major participants involved in the scleroderma diagnostics and therapeutics market on a global and regional basis.
Some of the major players in the global scleroderma diagnostics and therapeutics market include:
- Bayer AG; Boehringer Ingelheim International GmbH
- arGentis Pharmaceuticals, LLC
- Celgene Corp.
- Bristol-Myers Squibb Company
- F. Hoffman La-Roche Ltd.
- Corbus Pharmaceuticals Holdings
- Seattle Genetics, Inc.
- Kadmon Holdings, Inc.
- Emerald Health Pharmaceuticals
- and Prometic Life Sciences, Inc.
Global scleroderma diagnostics and therapeutics market is segmented as follows:
By Diagnostic Test Type
- Blood Tests
- Imaging Techniques
- Skin Biopsy
- Pulmonary Function Tests
- Electrocardiogram & Echocardiogram
By Indication
- Systemic Scleroderma
- Localized Scleroderma
By Drug Class
- Prostacyclin Analogues
- Endothelin Receptor Antagonists
- Phosphodiesterase 5 Inhibitors – PHA
- Immunosuppressors
- Analgesics
- Calcium Channel Blockers
- Others
By Region
- North America
- The U.S.
- Canada
- Mexico
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of Asia Pacific
- The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
Table Of Content
Methodology
FrequentlyAsked Questions
The global scleroderma diagnostics and therapeutics market is expected to grow due to increasing disease awareness, advancements in biomarker research, rising prevalence of autoimmune disorders, and growing demand for targeted therapies.
According to a study, the global scleroderma diagnostics and therapeutics market size was worth around USD 2.52 Billion in 2024 and is expected to reach USD 3.98 Billion by 2034.
The global scleroderma diagnostics and therapeutics market is expected to grow at a CAGR of 5.2% during the forecast period.
North America is expected to dominate the scleroderma diagnostics and therapeutics market over the forecast period.
Leading players in the global scleroderma diagnostics and therapeutics market include Bayer AG; Boehringer Ingelheim International GmbH, arGentis Pharmaceuticals, LLC, Celgene Corp., Bristol-Myers Squibb Company, F. Hoffman La-Roche Ltd., Corbus Pharmaceuticals Holdings, Seattle Genetics Inc., Kadmon Holdings Inc., Emerald Health Pharmaceu, among others.
The report explores crucial aspects of the scleroderma diagnostics and therapeutics market, including a detailed discussion of existing growth factors and restraints, while also examining future growth opportunities and challenges that impact the market.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed