U.S. Multi Cancer Early Detection Market Size, Share, Trends, Growth and Forecast 2032

U.S. Multi Cancer Early Detection Market By Type (Liquid Biopsy, LDT, and Gene Panel), By End-Use (Diagnostic Laboratories and Hospitals), and By Region - Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2024 - 2032-
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 1,000 Million | USD 1,335 Million | 6% | 2023 |
U.S. Multi Cancer Early Detection Industry Prospective:
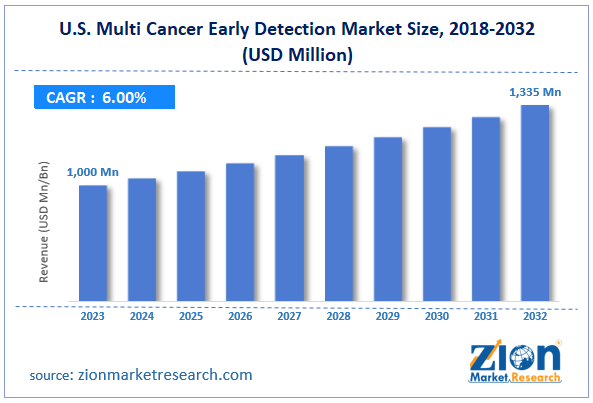
The U.S. multi cancer early detection market size was evaluated at $1,000 million in 2023 and is slated to hit $1,335 million by the end of 2032 with a CAGR of nearly 6% between 2024 and 2032.
U.S. Multi Cancer Early Detection Market: Overview
Multi-cancer early detection (MCED) is an evolving field in oncology that is aimed at detecting many kinds of cancer from a single test before appearance of symptoms. This approach impacts breakthroughs in genomics, biotechnology, and data analytics for enhancing early diagnostics, which can bring improvement in the treatment results and rate of survival.
Key Insights
- As per the analysis shared by our research analyst, the U.S. multi cancer early detection market is projected to expand annually at the annual growth rate of around 6% over the forecast timespan (2024-2032)
- In terms of revenue, the U.S. multi cancer early detection market size was evaluated at nearly $1,000 million in 2023 and is expected to reach $1,335 million by 2032.
- The global U.S. multi cancer early detection market is anticipated to grow rapidly over the forecast timeline owing to technological breakthroughs and large-scale use of AI/ML for detecting chronic ailments.
- In terms of type, the LDT segment is slated to register the highest CAGR over the forecast period.
- Based on end-use, the hospitals segment is predicted to dominate the segmental growth in the upcoming years.
- Region-wise, the Northeast multi cancer early detection industry in the U.S. is projected to register the fastest CAGR during the projected timespan.
 Request Free Sample
Request Free Sample
U.S. Multi Cancer Early Detection Market: Growth Factors
Massive use of AI for identifying cancer can boost the market growth trends in the U.S.
Technological breakthroughs and large-scale use of AI/ML for detecting chronic ailments will prompt the growth of the U.S. multi cancer early detection market. Surging cases of cancer and a rising geriatric population prone to the ailment can enlarge the scope of the market growth in the country. Growing consciousness about the early detection of cancer and the limits of recent screening procedures will embellish the demand for multi-cancer early detection tests, thereby driving regional market trends. Moreover, the growing preference of patients & healthcare service providers for non-invasive as well as comprehensive testing services for early cancer identification can pave the way for the immense growth of the market in the country. Favorable compensation guidelines & healthcare insurance coverage for multi-cancer early detection testing can boost the regional market trends. Reportedly, approvals of MCED tests by the U.S. FDA for improving its cost-efficiency will scale up the market expansion in the country.
U.S. Multi Cancer Early Detection Market: Restraints
Escalating costs of MCED products can disrupt the growth of the industry in the U.S.
Surging prices of MCED systems, protection of sensitive data, and regulatory hurdles can restrict the expansion of the U.S. multi cancer early detection industry. Growing data privacy issues as well as ethical issues can also put brakes on the industry surge in the country.
U.S. Multi Cancer Early Detection Market: Opportunities
Escalating use of precision medicine to create new growth facets for the market growth in the U.S.
Growing demand for personalized medicine is likely to open new growth opportunities for the U.S. multi cancer early detection market. Breakthroughs in genomics and routine screening as well as primary care practices are anticipated to spur the expansion of the market in the U.S.
U.S. Multi Cancer Early Detection Market: Challenges
Exponential rate of diagnostics costs can create hiccups in the growth of the industry in the U.S.
Surging diagnostic charges and less awareness about MCED in emerging economies can challenge the growth of the U.S. multi cancer early detection industry. Necessity of high investments for procuring the product can impede the industry growth in the country.
U.S. Multi Cancer Early Detection Market: Segmentation
The U.S. multi cancer early detection market is divided into type, end-use, and region.
In terms of type, the U.S. multi cancer early detection market is bifurcated into liquid biopsy, LDT, and gene panel segments. Additionally, the LDT segment, which gained approximately half of the market revenue share in 2023, is expected to record the fastest rate of growth each year during the time interval from 2024 to 2032. The main factor fostering the segmental surge can be due to the lack of U.S. FDA approval for the use of LDT in labs.
On the basis of end-use, the U.S. multi cancer early detection industry is sectored into diagnostic laboratories and hospitals segments. In addition to this, the hospitals segment, which accrued about 53% of the industry share in 2023, is set to lead the segmental expansion in the U.S. in the upcoming years. The segmental expansion can be due to a large-scale preference for MCED tests in hospitals. Reportedly, hospitals can provide these tests to people even in case of exigencies.
U.S. Multi Cancer Early Detection Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | U.S. Multi Cancer Early Detection Market |
| Market Size in 2023 | USD 1,000 Million |
| Market Forecast in 2032 | USD 1,335 Million |
| Growth Rate | CAGR of 6% |
| Number of Pages | 222 |
| Key Companies Covered | Laboratory for Advanced Medicine Inc., Micronoma Inc., Freenome Holdings Inc., Illumina Inc., Guardant Health, Elypta AB, Exact Sciences Corporation, VESEN Inc., ClearNote Health, Burning Rock Biotech Limited, GENECAST, FOUNDATION MEDICINE INC., Oncocyte Corporation, ADELA Inc., SeekIn., and others. |
| Segments Covered | By Type, By Application, and By Region |
| Regions Covered in U.S. | Northeast, Midwest, South, and West |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
U.S. Multi Cancer Early Detection Market: Regional Insights
Southern region of the U.S. is expected to lead the market surge over 2024-2032
The Southern region, which accrued 29% of the U.S. multi cancer early detection market share in 2023, is set to retain its position in the U.S. market in the upcoming years. In addition to this, the regional market expansion in the forecast timeline can be attributed to the escalating number of cancer diagnoses in people belonging to this part of the country. For the record, more than 1 million people are diagnosed with cancer in the U.S., and the southern part of the country accounts for a large portion of the cases. Favorable initiatives by the government in the southern states of the U.S. Surging awareness about the healthcare and benefits of regular health checkups will drive the expansion of the market in the Southern part of the U.S. Presence of strong healthcare infrastructure and fund allocation for research activities will proliferate the size of the market in the South U.S.
The Northeast multi cancer early detection industry in the U.S. is predicted to record the highest rate of expansion annually within the next few years. The growth of the industry in the Northeastern part of the U.S. can be due to the flourishing pharmaceuticals & life-science sectors. An increase in the customer spending on healthcare will drive the industry growth in the region. Presence of giant MCED manufacturers will drive the growth of the industry in this part of the U.S.
U.S. Multi Cancer Early Detection Market: Competitive Space
The U.S. multi cancer early detection market profiles key players such as:
- Laboratory for Advanced Medicine Inc.
- Micronoma Inc.
- Freenome Holdings Inc.
- Illumina Inc.
- Guardant Health
- Elypta AB
- Exact Sciences Corporation
- VESEN Inc.
- ClearNote Health
- Burning Rock Biotech Limited
- GENECAST
- FOUNDATION MEDICINE INC.
- Oncocyte Corporation
- ADELA Inc.
- SeekIn.
The U.S. multi cancer early detection market is segmented as follows:
By Type
- Liquid Biopsy
- LDT
- Gene Panel
By Application
- Diagnostic Laboratories
- Hospitals
By Region
The U.S.
- Northeast
- Midwest
- South
- West
Table Of Content
Methodology
FrequentlyAsked Questions
Multi-cancer early detection (MCED) is an evolving field in oncology that aims to detect many kinds of cancer from a single test before symptoms appear.
The U.S. multi-cancer early detection market growth over the forecast period can be owing to surging cases of cancer and a rising geriatric population prone to the ailment.
According to a study, the global U.S. multi cancer early detection industry size was $1,000 million in 2023 and is projected to reach $1,335 million by the end of 2032.
The global U.S multi-cancer early detection market is anticipated to record a CAGR of nearly 6% from 2024 to 2032.
The Northeast multi-cancer early detection industry of the U.S. is set to register the fastest CAGR over the forecasting timeline owing to flourishing pharmaceuticals and life-science sectors. An increase in customer spending on healthcare will drive industry growth in the region. The presence of giant MCED manufacturers will also drive the growth of the industry in this part of the U.S.
The U.S. multi-cancer early detection market is led by players such as Laboratory for Advanced Medicine, Inc., Micronoma Inc., Freenome Holdings, Inc., Illumina, Inc., Guardant Health, Elypta AB, Exact Sciences Corporation, VESEN, Inc., ClearNote Health, Burning Rock Biotech Limited, GENECAST, FOUNDATION MEDICINE, INC., Oncocyte Corporation, ADELA, Inc., and SeekIn.
The U.S. multi-cancer early detection market report covers the geographical market along with a comprehensive competitive landscape analysis. It also includes cash flow analysis, profit ratio analysis, market basket analysis, market attractiveness analysis, sentiment analysis, PESTEL analysis, trend analysis, SWOT analysis, trade area analysis, demand & supply analysis, Porter’s five force analysis, and value chain analysis.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed