Pharmacovigilance and Drug Safety Software Market Size, Share, And Growth Report 2032

Pharmacovigilance and Drug Safety Software Market by Functionality (ADR Reporting Software, Drug Safety Audits Software, Issue Tracking Software, and Fully Integrated Software), by Delivery Mode (On-Premises and On-Demand), by End-User (Pharma & Biotech Companies, Contract Research Organizations (CROS), Business Process Outsourcing (BPO) Firms, and Other Pharmacovigilance Service Providers), and By Region - Global And Regional Industry Overview, market Intelligence, Comprehensive Analysis, Historical Data, And Forecasts 2024-2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 200.5 Million | USD 370.48 Million | 7.06% | 2023 |
Pharmacovigilance and Drug Safety Software Market: Size
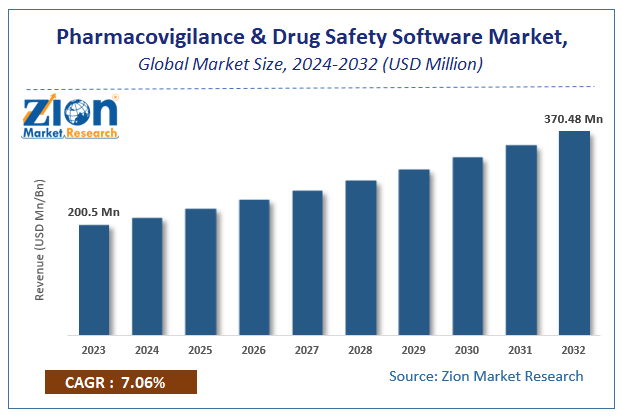
The global Pharmacovigilance and Drug Safety Software Market size was worth around USD 200.5 million in 2023 and is predicted to grow to around USD 370.48 million by 2032 with a compound annual growth rate (CAGR) of roughly 7.06% between 2024 and 2032.
The study provides historical data from 2018 to 2022 along with a forecast from 2024 to 2032 based on revenue (USD million). The report covers a forecast and an analysis of the Pharmacovigilance and Drug Safety Software Market on a global and regional level.
Pharmacovigilance and Drug Safety Software Market: Overview
The scope and definition of pharmacovigilance have evolved to identify the significance of a systems approach for improving and monitoring the harmless and safe use of medicines. According to the World Health Organization (WHO), pharmacovigilance is the science and activities related to the understanding, evaluation, detection, and prevention of adversarial reactions to medications or problems related to medicine. This pharmacovigilance software is majorly used by biotech and pharmaceutical organizations, business process outsourcing firms (BPOs), contract research organizations (CROs), and many other pharmacovigilance service providers.
Pharmacovigilance globalization with extensive availability and access of the internet is likely to fuel the global pharmacovigilance and drug safety software market in the future. Moreover, the rising health information functionality will further support the adoption and usage of pharmacovigilance software over the estimated period, as it reduces medical expenditures and improves patient health. The augmenting adoption of pharmacovigilance software by numerous outsourcing companies will also serve as a huge growth opportunity for the global pharmacovigilance and drug safety software market over the forecast timespan. In contrast, government bodies comprising EMEA and FDA have increased the pressure on pharmaceutical and biotechnology companies for manufacturing safe drugs.
Pharmacovigilance and Drug Safety Software Market: Segmentation
The study provides a decisive view of the pharmacovigilance and drug safety software market by segmenting the market based on functionality, delivery mode, end-user, and region. All the segments have been analyzed based on present and future trends and the market is estimated from 2024 to 2032.
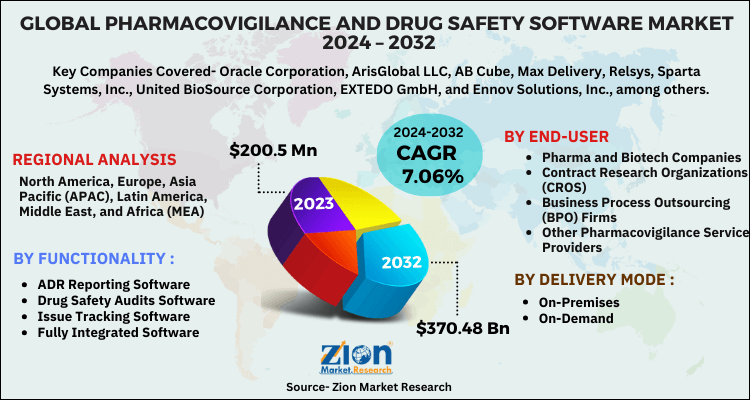
Based on functionality, the market is segmented into drug safety audits software, ADR reporting software, fully integrated software, and issue tracking software. The fully integrated software segment is expected to register the highest growth over the forecast time period, owing to the increasing need to prevent errors in database management regarding medicines.
By delivery mode, the market is segmented into on-premises and on-demand. The on-premises segment is anticipated to hold the largest market share, whereas, the on-demand segment is expected to register the highest CAGR over the estimated time period.
By end-user, the market is segmented into contract research organizations (CROS), business process outsourcing (BPO) firms, pharma and biotech companies, and other pharmacovigilance service providers.
Pharmacovigilance and Drug Safety Software Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Pharmacovigilance and Drug Safety Software Market |
| Market Size in 2023 | USD 200.5 Million |
| Market Forecast in 2032 | USD 370.48 Million |
| Growth Rate | CAGR of 7.06% |
| Number of Pages | 110 |
| Key Companies Covered | Oracle Corporation, ArisGlobal LLC, AB Cube, Max Delivery, Relsys, Sparta Systems, Inc., United BioSource Corporation, EXTEDO GmbH, and Ennov Solutions, Inc., among others |
| Segments Covered | By Functionality, By Delivery Mode,By End-User and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Pharmacovigilance and Drug Safety Software Market: Regional Analysis
The regional segment includes the current and forecast demand for North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa with its further segmentation into major countries including the U.S., Germany, France, UK, China, Japan, India, and Brazil.
By region, North America is anticipated to lead the global pharmacovigilance and drug safety software market over the forecast period, owing to the high adoption of pharmacovigilance and drug safety software and favorable government regulations. Europe was the second largest market in 2023, followed by the Asia Pacific. The Asia Pacific pharmacovigilance and drug safety software market is anticipated to record lucrative growth over the projected time period, subject to an increasing number of clinical trials being conducted in the region and the considerable cost-saving benefits.
Pharmacovigilance and Drug Safety Software Market: Competitive Players
Some key players of the global pharmacovigilance and drug safety software market include:
- Oracle Corporation
- ArisGlobal LLC
- AB Cube
- Max Delivery
- Relsys
- Sparta Systems Inc.
- United BioSource Corporation
- EXTEDO GmbH
- Ennov Solutions Inc.
The Global Pharmacovigilance and Drug Safety Software Market is segmented as follows:
Global Pharmacovigilance and Drug Safety Software Market: Functionality Analysis
- ADR Reporting Software
- Drug Safety Audits Software
- Issue Tracking Software
- Fully Integrated Software
Global Pharmacovigilance and Drug Safety Software Market: Delivery Mode Analysis
- On-Premises
- On-Demand
Global Pharmacovigilance and Drug Safety Software Market: End-User Analysis
- Pharma and Biotech Companies
- Contract Research Organizations (CROS)
- Business Process Outsourcing (BPO) Firms
- Other Pharmacovigilance Service Providers
Global Pharmacovigilance and Drug Safety Software Market: Regional Analysis
- North America
- The U.S.
- Europe
- UK
- France
- Germany
- Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- Middle East and Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Pharmacovigilance and drug safety software or tools aid in classifying, reviewing, and forming pharmacovigilance data. This software also helps in the formation of repots related to adverse medical events.
According to study, the Pharmacovigilance and Drug Safety Software Market size was worth around USD 200.5 million in 2023 and is predicted to grow to around USD 370.48 million by 2032.
The CAGR value of Pharmacovigilance and Drug Safety Software Market is expected to be around 7.06% during 2024-2032.
North America has been leading the Pharmacovigilance and Drug Safety Software Market and is anticipated to continue on the dominant position in the years to come.
Which are the major players leveraging the Pharmacovigilance and Drug Safety Software Market growth?
The Pharmacovigilance and Drug Safety Software Market is led by players like Oracle Corporation, ArisGlobal LLC, AB Cube, Max Delivery, Relsys, Sparta Systems Inc., United BioSource Corporation, EXTEDO GmbH, Ennov Solutions Inc., and among others.
Choose License Type
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed