Phenylketonuria Treatment Market Size, Share, And Growth Report 2032

Phenylketonuria Treatment Market By Type (Drugs [Kuvan, and Biopten], and Dietary Supplements), By Route of Administration (Oral and Parenteral), By End User (Hospital Pharmacies, Drug Stores, Online Pharmacies, Pediatric Clinics, and Others), By Region - Global And Regional Industry Overview, market Intelligence, Comprehensive Analysis, Historical Data, And Forecasts 2024-2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 863.9 Million | USD 1372.7 Million | 5.28% | 2023 |
Phenylketonuria Treatment Market Size
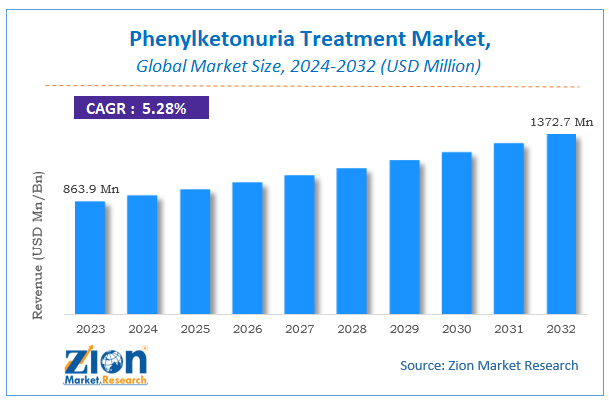
The global Phenylketonuria Treatment market size was worth around USD 863.9 million in 2023 and is predicted to grow to around USD 1372.7 million by 2032 with a compound annual growth rate (CAGR) of roughly 5.28% between 2024 and 2032.
The study provides historical data from 2018 to 2022 along with a forecast from 2024 to 2032 based on revenue (USD million). The report covers a forecast and an analysis of the Phenylketonuria Treatment market on a global and regional level.
Phenylketonuria Treatment Market: Overview
Phenylketonuria or PKU is a genetic disorder in which the levels of phenylalanine increase in the blood. Phenylalanine is a building block of protein obtained through food. PKU can cause behavioral problems, seizures, psychiatric disorders, and delayed development if not treated on time. Children with classic PKU are at risk of brain damage and require treatment. PKU varies from mild to severe form, however classic PKU is considered as severe and variant PKU is a less severe disease form. The rising prevalence of PKU globally is likely to accelerate the market growth for PKU treatment. As per the stats provided by Genetic Home Reference, in the U.S. 1 in 10,000 to 15,000 cases of PKU occur in newborns.
In-depth secondary research is used to ascertain overall market size, top industry players, top products, industry associations, etc. Macro-economic indicators such as healthcare industry outlook, healthcare spending, research funding, GDP along with company websites, company annual reports, white papers, financial reports, and other sources have also been considered to arrive at the indicated market numbers.
The study also includes drivers and restraints for the phenylketonuria treatment along with the impact they have on the demand over the forecast period. Besides, the report includes the study of opportunities and trends available in the phenylketonuria treatment market on a global level. The presence of a large number of pipeline drugs, like the SYNB1618, RTX-134, and CNSA-001 are likely to fuel the market growth for phenylketonuria treatment. CNSA-001 has successfully completed its Phase I study and has initiated a Phase II clinical trial whereas SYNB1618 is currently under Phase 2a clinical trial and has received the FDA’s fast-track designation. Besides, rising commercialization and development of new drugs are likely to boost market growth.
Global Phenylketonuria Treatment Market: Segmentation
The study provides a decisive view of the phenylketonuria treatment market by segmenting the market based on type, route of administration, end user, and regions. All the segments have been analyzed based on present and future trends and the market is estimated from 2024 to 2032.
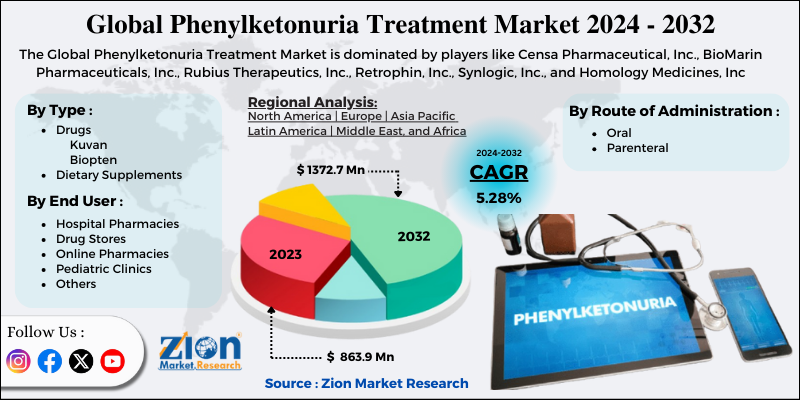
Based on type the market is segmented into drugs and dietary supplements. The drug market for phenylketonuria is further segmented into kuvan, and biopterin. The Kuvan segment accounted for a major share of the market in 2023. BioMarin Pharmaceutical, Inc. manufactured the oral drug Kuvan. This drug was approved in 2007 in the U.S. and in Europe in 2008. The patent of this drug expired in the year 2015 in the U.S. and is about to expire in the year 2020 in Europe. Nonetheless, BioMarin has entered into a settlement agreement with Par Pharmaceuticals to launch a generic version of Kuvan tablets and powder form.
Based on route of administration the market is segmented into oral, and parenteral. The oral route of administration held the major share of the market in 2018 since this route is mainly preferred by patients due to its ease of administration and convenience. Besides, parenteral infusions are also difficult to administer.
Based on end-user the market is segmented into hospital pharmacies, drug stores, online pharmacies, pediatric clinics, and others. The hospital pharmacies segment held the major share of the market in 2023.
Phenylketonuria Treatment Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Phenylketonuria Treatment Market |
| Market Size in 2023 | USD 863.9 Million |
| Market Forecast in 2032 | USD 1372.7 Million |
| Growth Rate | CAGR of 5.28% |
| Number of Pages | 110 |
| Key Companies Covered | Censa Pharmaceutical, Inc., BioMarin Pharmaceuticals, Inc., Rubius Therapeutics, Inc., Retrophin, Inc., Synlogic, Inc., and Homology Medicines, Inc |
| Segments Covered | By Type, By Route Of Administration, By Application and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Phenylketonuria Treatment Market: Regional Analysis
Regional segmentation includes the current and forecast demand for the Asia Pacific, North America, Latin America, Europe, and Middle East & Africa with its further bifurcation into major countries. North America held the major share of the market owing to rising government initiatives and favorable regulations to treat PKU in this region. Besides, continuous R&D and commercialization of novel drugs are likely to propel PKU treatment market growth in this region.
Global Phenylketonuria Treatment Market: Competitive Players
The report also provides a company market share analysis in order to give a broader view of the key players in the market. Industry insights and information is delivered in the required format. ZMR develops a list of industry players (manufacturers), distributors, retailers, and industry experts. Some of the players included in the phenylketonuria treatment market are:
- Censa Pharmaceutical
- BioMarin Pharmaceuticals
- Rubius Therapeutics
- Retrophin
- Synlogic
- Homology Medicines
Phenylketonuria Treatment Market: Competitive Players
Global Phenylketonuria Treatment Market: Type Segment Analysis
- Drugs
- Kuvan
- Biopten
- Dietary Supplements
Global Phenylketonuria Treatment Market: Route of Administration Segment Analysis
- Oral
- Parenteral
Global Phenylketonuria Treatment Market: End User Segment Analysis
- Hospital Pharmacies
- Drug Stores
- Online Pharmacies
- Pediatric Clinics
- Others
Global Phenylketonuria Treatment Market: Regional Segment Analysis
-
North America
- The U.S.
- Europe
- UK
- France
- Germany
- Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- The Middle East and Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Phenylketonuria (PKU) treatment focuses on managing the levels of phenylalanine, an amino acid that accumulates due to a genetic defect in the enzyme phenylalanine hydroxylase.
According to study, the Phenylketonuria Treatment Market size was worth around USD 863.9 million in 2023 and is predicted to grow to around USD 1372.7 million by 2032.
The CAGR value of Phenylketonuria Treatment Market is expected to be around 5.28% during 2024-2032.
North America has been leading the Phenylketonuria Treatment Market and is anticipated to continue on the dominant position in the years to come.
The Phenylketonuria Treatment Market is led by players like Censa Pharmaceutical Inc., BioMarin Pharmaceuticals Inc., Rubius Therapeutics Inc., Retrophin Inc., Synlogic Inc., and Homology Medicines Inc.
Choose License Type
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed